| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5222826 | Tetrahedron | 2009 | 10 Pages |
Abstract
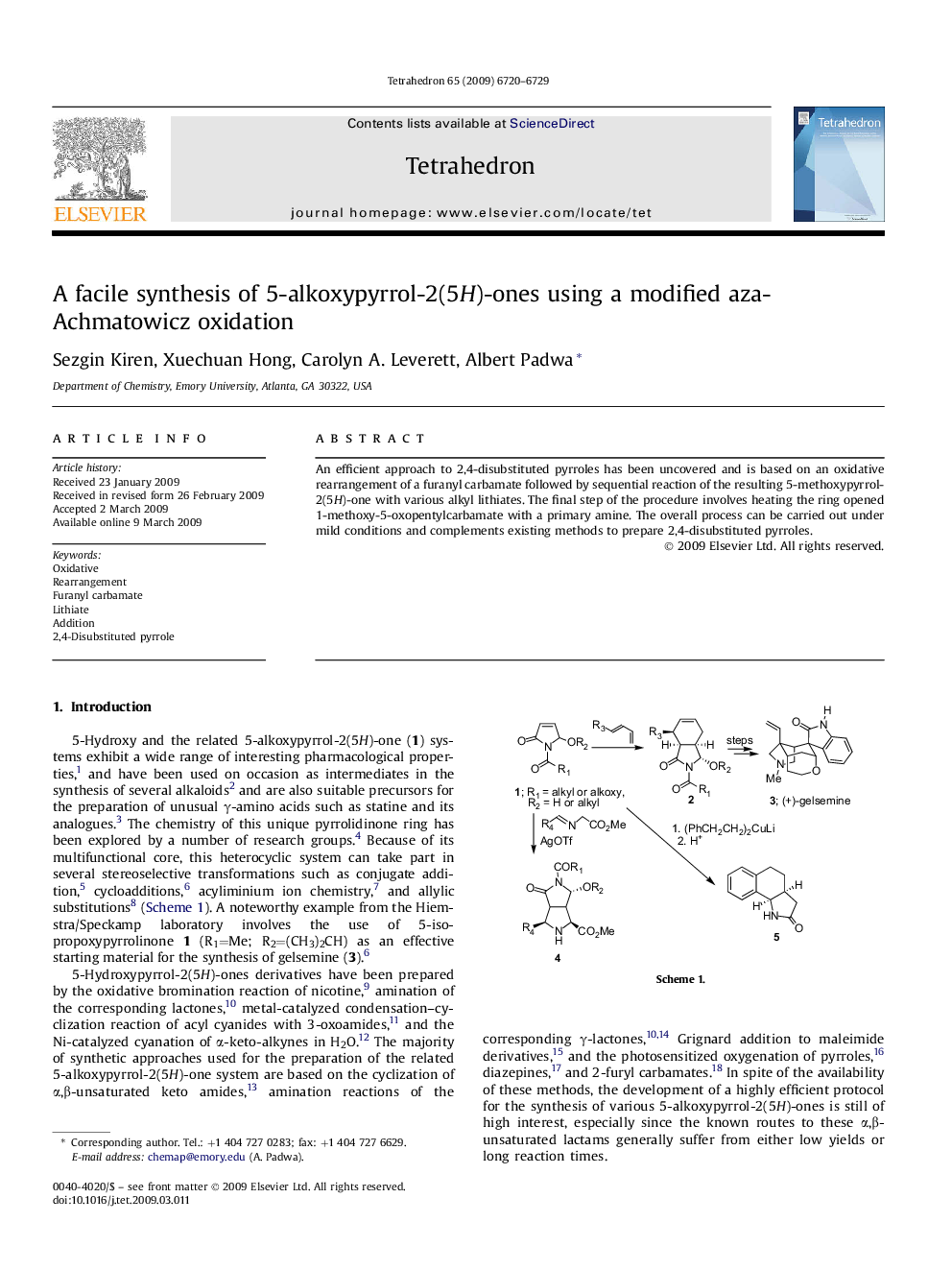
An efficient approach to 2,4-disubstituted pyrroles has been uncovered and is based on an oxidative rearrangement of a furanyl carbamate followed by sequential reaction of the resulting 5-methoxypyrrol-2(5H)-one with various alkyl lithiates. The final step of the procedure involves heating the ring opened 1-methoxy-5-oxopentylcarbamate with a primary amine. The overall process can be carried out under mild conditions and complements existing methods to prepare 2,4-disubstituted pyrroles.
Graphical abstractDownload full-size image
Keywords
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry
Authors
Sezgin Kiren, Xuechuan Hong, Carolyn A. Leverett, Albert Padwa,