| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5223395 | Tetrahedron | 2010 | 5 Pages |
Abstract
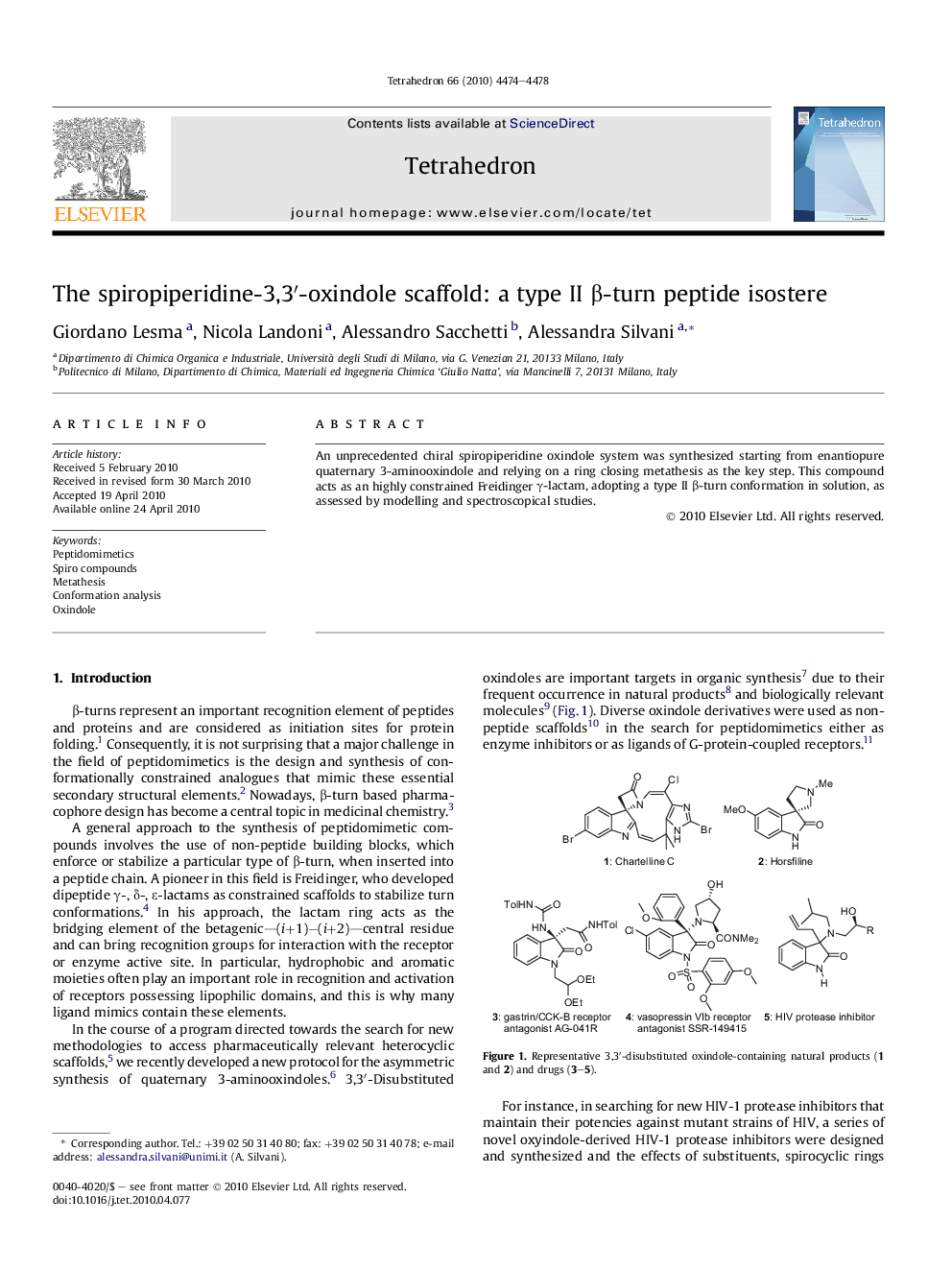
An unprecedented chiral spiropiperidine oxindole system was synthesized starting from enantiopure quaternary 3-aminooxindole and relying on a ring closing metathesis as the key step. This compound acts as an highly constrained Freidinger γ-lactam, adopting a type II β-turn conformation in solution, as assessed by modelling and spectroscopical studies.
Graphical abstractDownload full-size image
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry
Authors
Giordano Lesma, Nicola Landoni, Alessandro Sacchetti, Alessandra Silvani,