| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5223663 | Tetrahedron | 2010 | 9 Pages |
Abstract
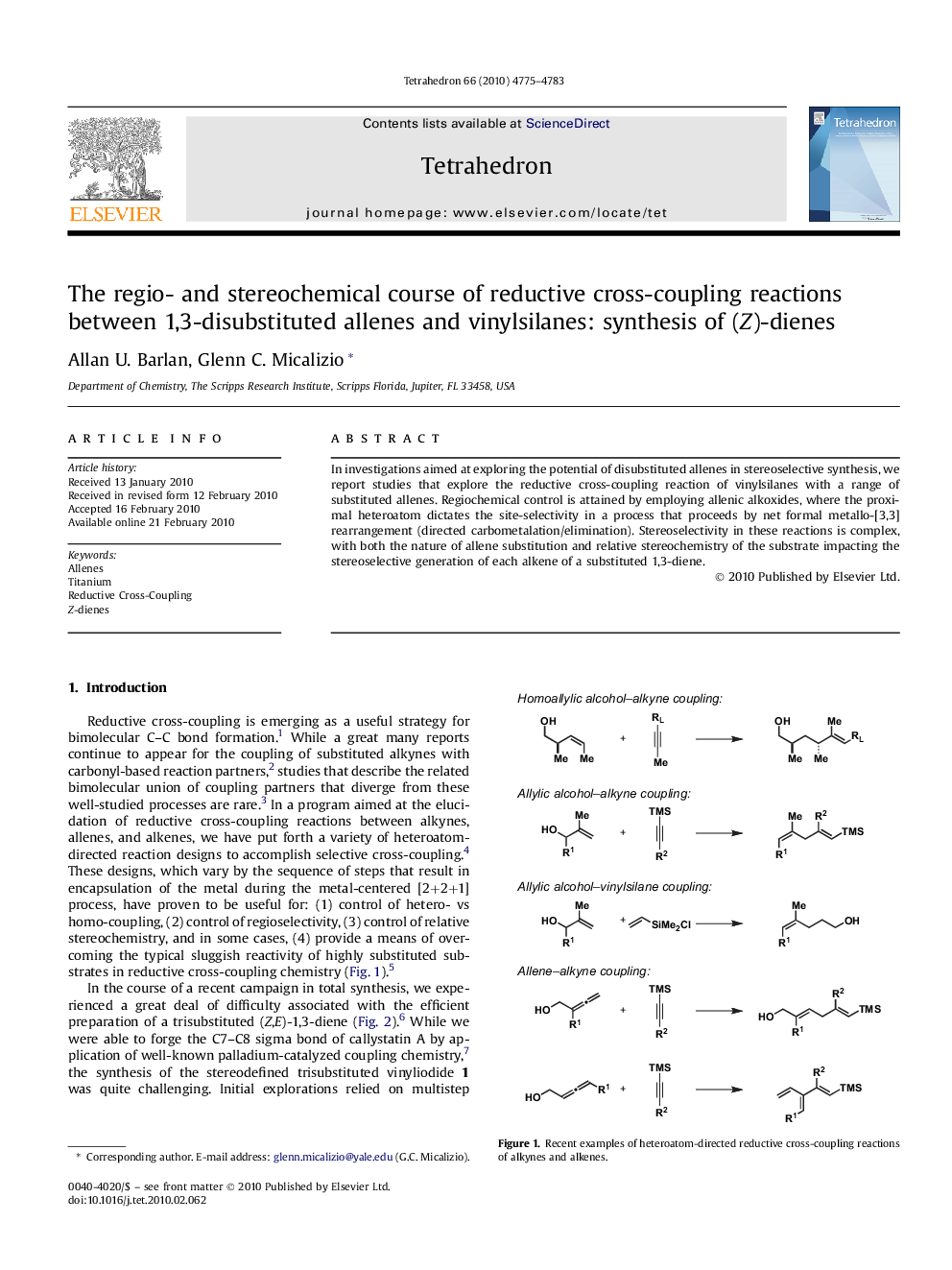
In investigations aimed at exploring the potential of disubstituted allenes in stereoselective synthesis, we report studies that explore the reductive cross-coupling reaction of vinylsilanes with a range of substituted allenes. Regiochemical control is attained by employing allenic alkoxides, where the proximal heteroatom dictates the site-selectivity in a process that proceeds by net formal metallo-[3,3] rearrangement (directed carbometalation/elimination). Stereoselectivity in these reactions is complex, with both the nature of allene substitution and relative stereochemistry of the substrate impacting the stereoselective generation of each alkene of a substituted 1,3-diene.
Graphical abstractDownload full-size image
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry
Authors
Allan U. Barlan, Glenn C. Micalizio,