| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5224248 | Tetrahedron | 2008 | 4 Pages |
Abstract
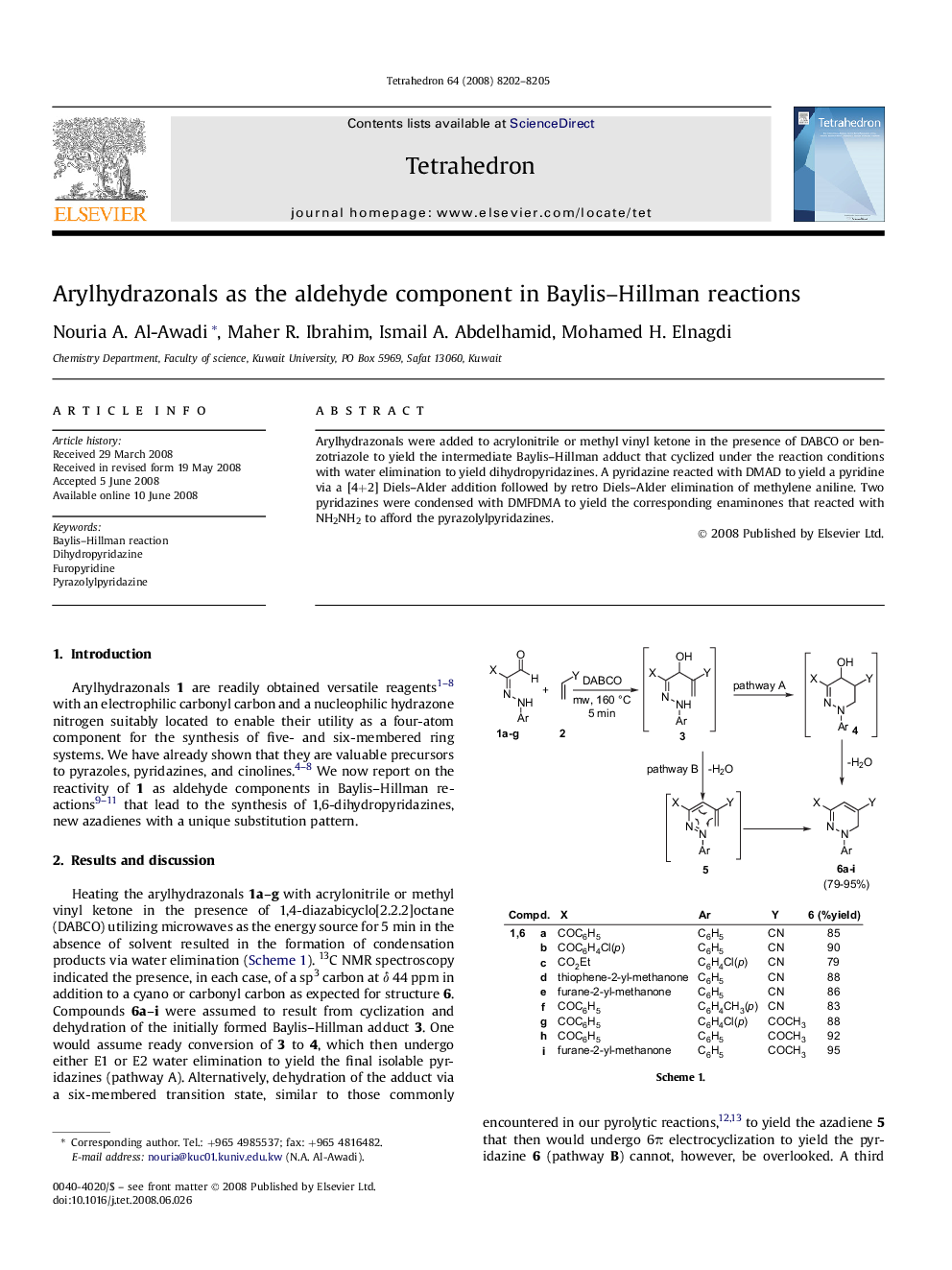
Arylhydrazonals were added to acrylonitrile or methyl vinyl ketone in the presence of DABCO or benzotriazole to yield the intermediate Baylis-Hillman adduct that cyclized under the reaction conditions with water elimination to yield dihydropyridazines. A pyridazine reacted with DMAD to yield a pyridine via a [4+2] Diels-Alder addition followed by retro Diels-Alder elimination of methylene aniline. Two pyridazines were condensed with DMFDMA to yield the corresponding enaminones that reacted with NH2NH2 to afford the pyrazolylpyridazines.
Graphical abstractDownload full-size image
Keywords
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry
Authors
Nouria A. Al-Awadi, Maher R. Ibrahim, Ismail A. Abdelhamid, Mohamed H. Elnagdi,