| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5225051 | Tetrahedron | 2007 | 9 Pages |
Abstract
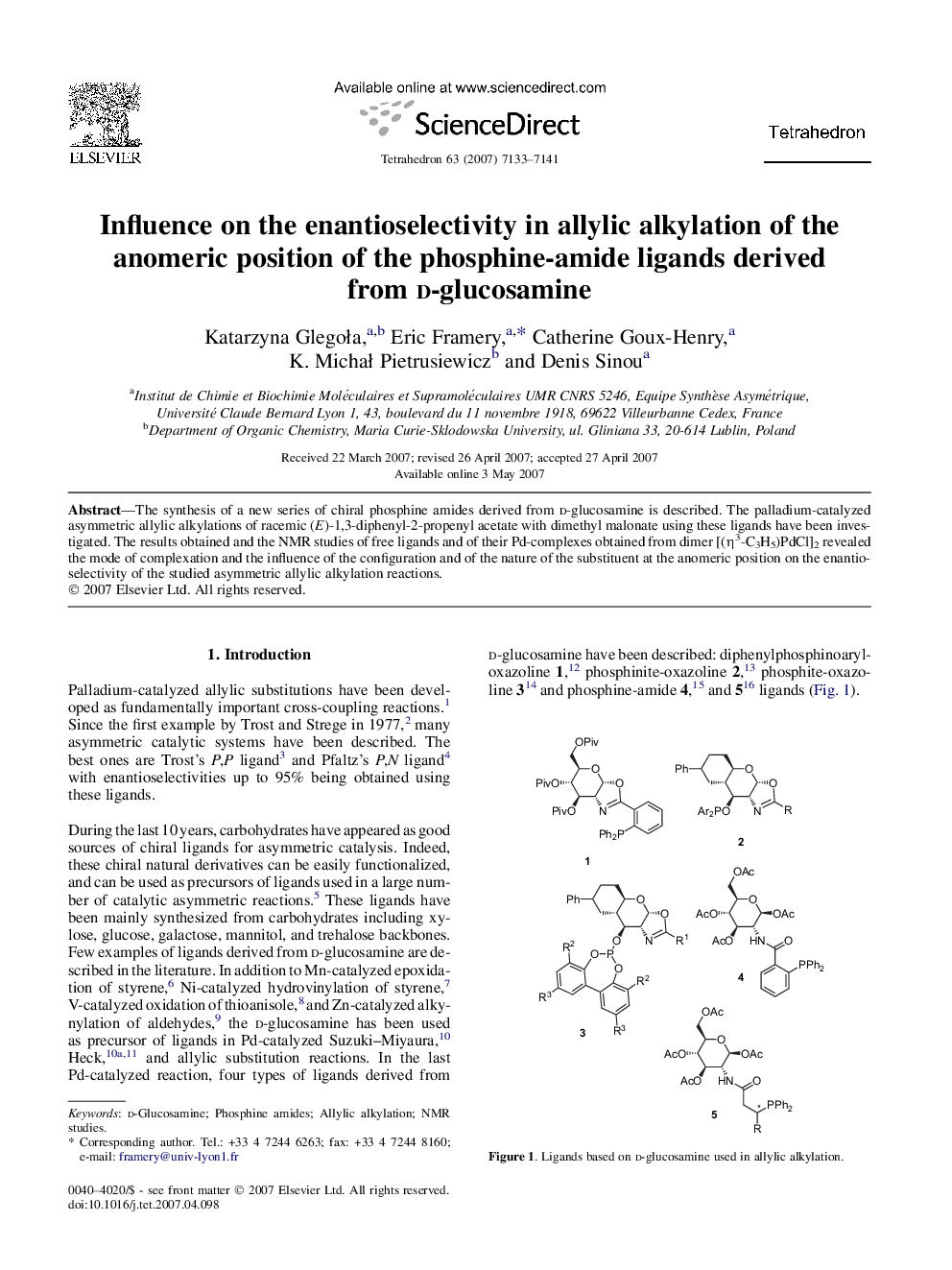
The synthesis of a new series of chiral phosphine amides derived from d-glucosamine is described. The palladium-catalyzed asymmetric allylic alkylations of racemic (E)-1,3-diphenyl-2-propenyl acetate with dimethyl malonate using these ligands have been investigated. The results obtained and the NMR studies of free ligands and of their Pd-complexes obtained from dimer [(η3-C3H5)PdCl]2 revealed the mode of complexation and the influence of the configuration and of the nature of the substituent at the anomeric position on the enantioselectivity of the studied asymmetric allylic alkylation reactions.
Graphical abstractDownload full-size image
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry
Authors
Katarzyna GlegoÅa, Eric Framery, Catherine Goux-Henry, K. MichaÅ Pietrusiewicz, Denis Sinou,