| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5226962 | Tetrahedron | 2007 | 8 Pages |
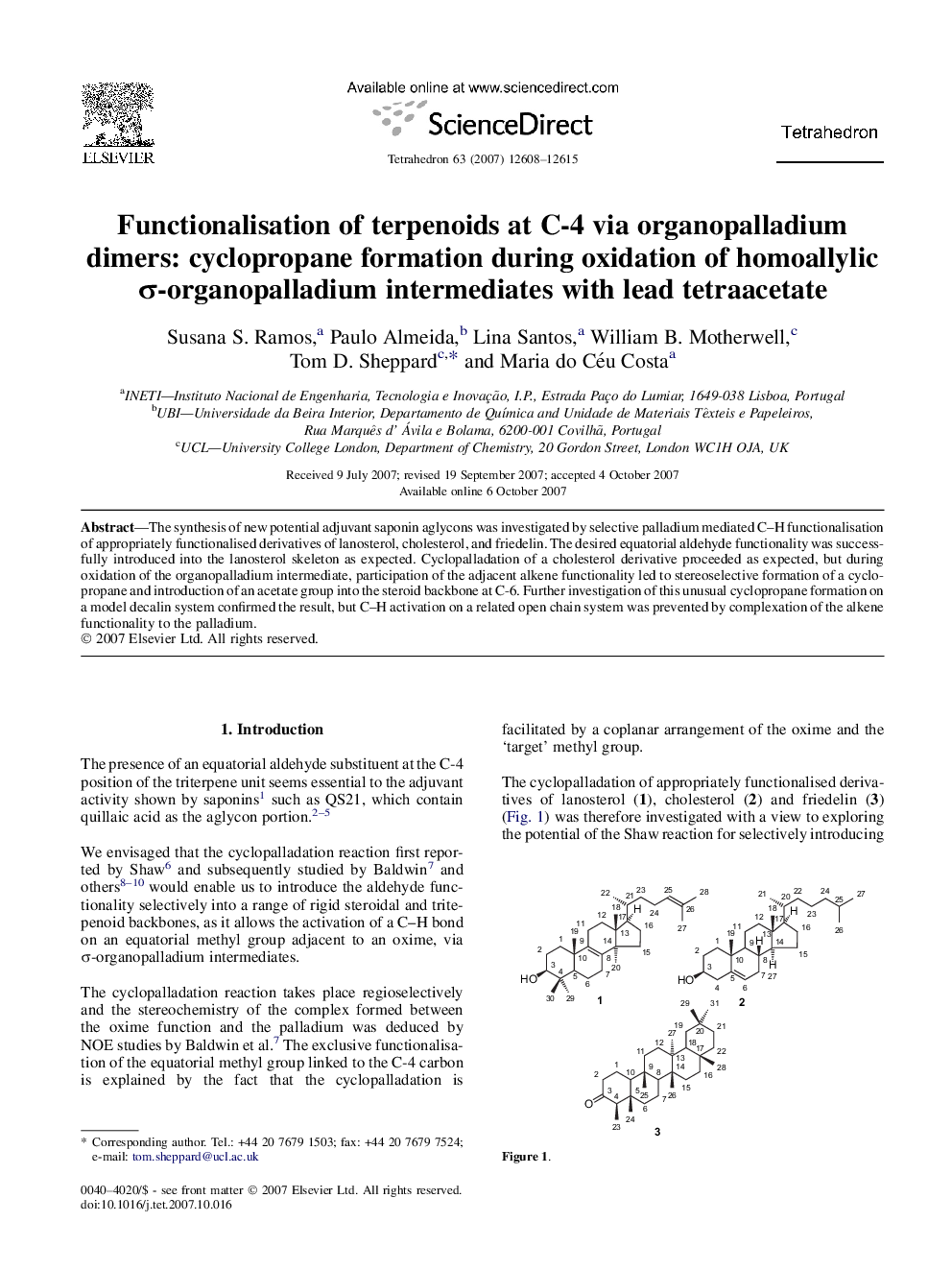
The synthesis of new potential adjuvant saponin aglycons was investigated by selective palladium mediated C-H functionalisation of appropriately functionalised derivatives of lanosterol, cholesterol, and friedelin. The desired equatorial aldehyde functionality was successfully introduced into the lanosterol skeleton as expected. Cyclopalladation of a cholesterol derivative proceeded as expected, but during oxidation of the organopalladium intermediate, participation of the adjacent alkene functionality led to stereoselective formation of a cyclopropane and introduction of an acetate group into the steroid backbone at C-6. Further investigation of this unusual cyclopropane formation on a model decalin system confirmed the result, but C-H activation on a related open chain system was prevented by complexation of the alkene functionality to the palladium.
Graphical abstract