| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5229574 | Tetrahedron | 2007 | 12 Pages |
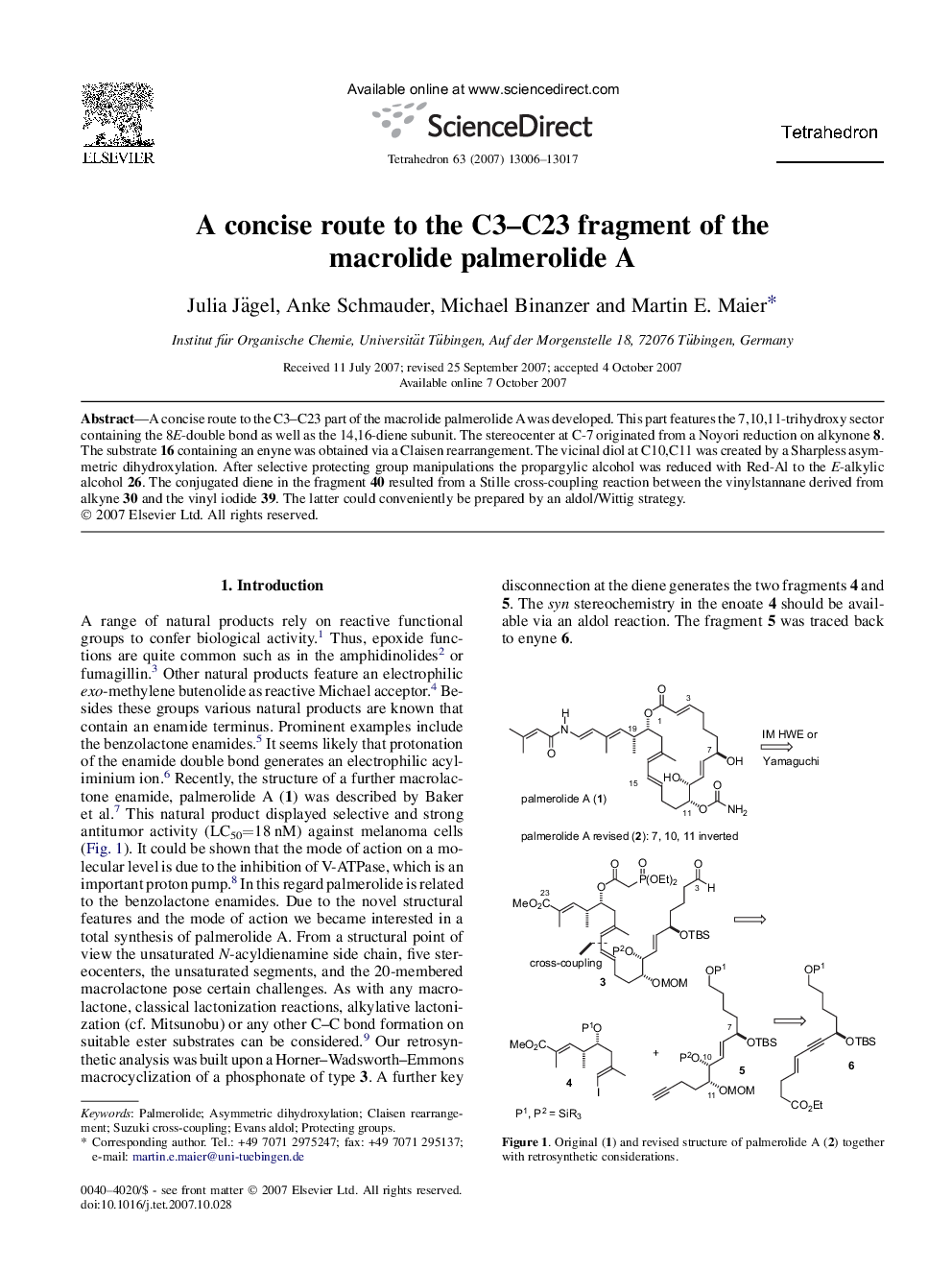
A concise route to the C3-C23 part of the macrolide palmerolide A was developed. This part features the 7,10,11-trihydroxy sector containing the 8E-double bond as well as the 14,16-diene subunit. The stereocenter at C-7 originated from a Noyori reduction on alkynone 8. The substrate 16 containing an enyne was obtained via a Claisen rearrangement. The vicinal diol at C10,C11 was created by a Sharpless asymmetric dihydroxylation. After selective protecting group manipulations the propargylic alcohol was reduced with Red-Al to the E-alkylic alcohol 26. The conjugated diene in the fragment 40 resulted from a Stille cross-coupling reaction between the vinylstannane derived from alkyne 30 and the vinyl iodide 39. The latter could conveniently be prepared by an aldol/Wittig strategy.
Graphical abstractDownload full-size image