| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5233657 | Tetrahedron | 2005 | 10 Pages |
Abstract
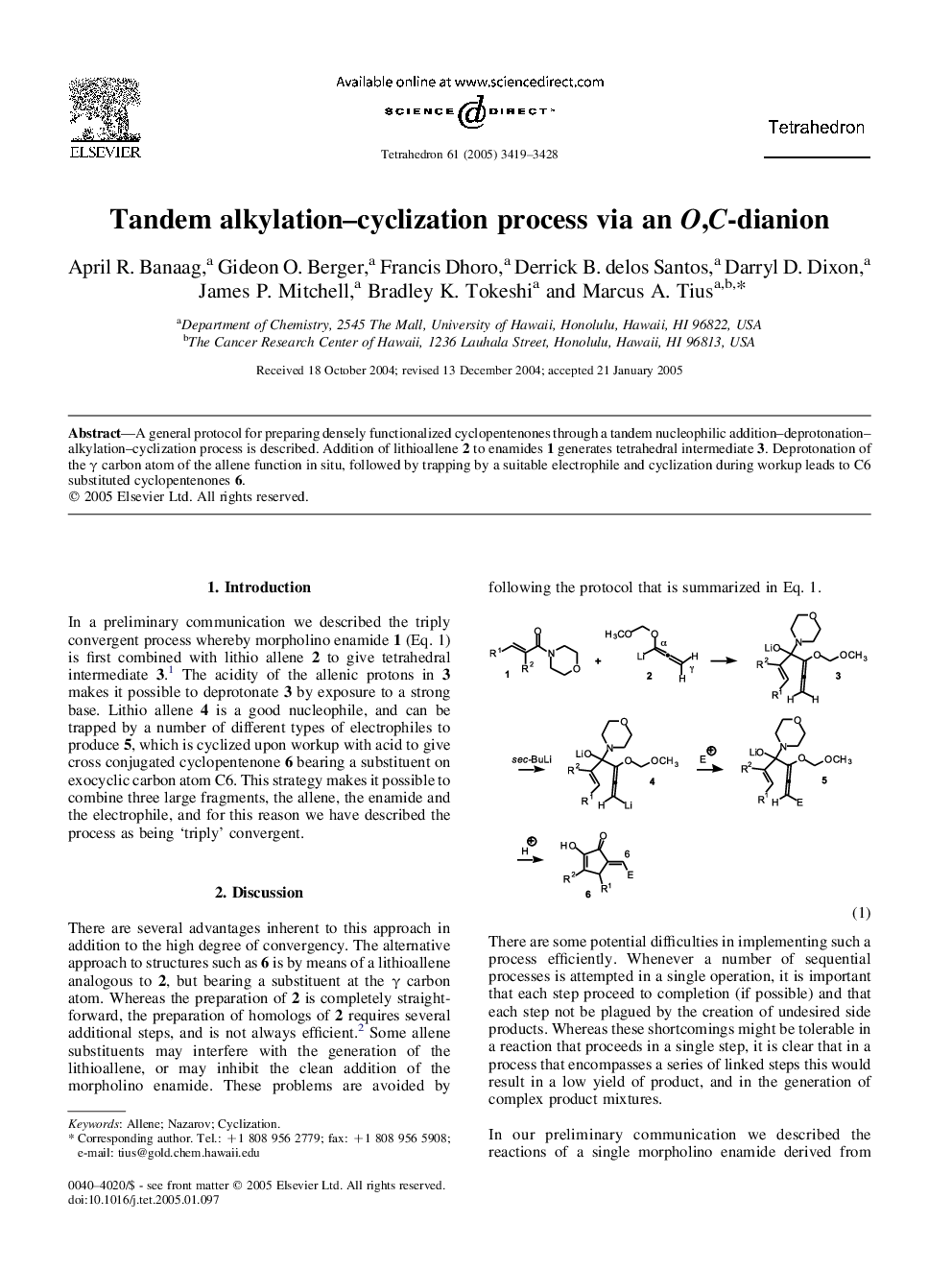
A general protocol for preparing densely functionalized cyclopentenones through a tandem nucleophilic addition-deprotonation-alkylation-cyclization process is described. Addition of lithioallene 2 to enamides 1 generates tetrahedral intermediate 3. Deprotonation of the γ carbon atom of the allene function in situ, followed by trapping by a suitable electrophile and cyclization during workup leads to C6 substituted cyclopentenones 6.
Graphical AbstractDownload full-size image
Keywords
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry
Authors
April R. Banaag, Gideon O. Berger, Francis Dhoro, Derrick B. delos Santos, Darryl D. Dixon, James P. Mitchell, Bradley K. Tokeshi, Marcus A. Tius,