| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5264553 | Tetrahedron Letters | 2017 | 5 Pages |
Abstract
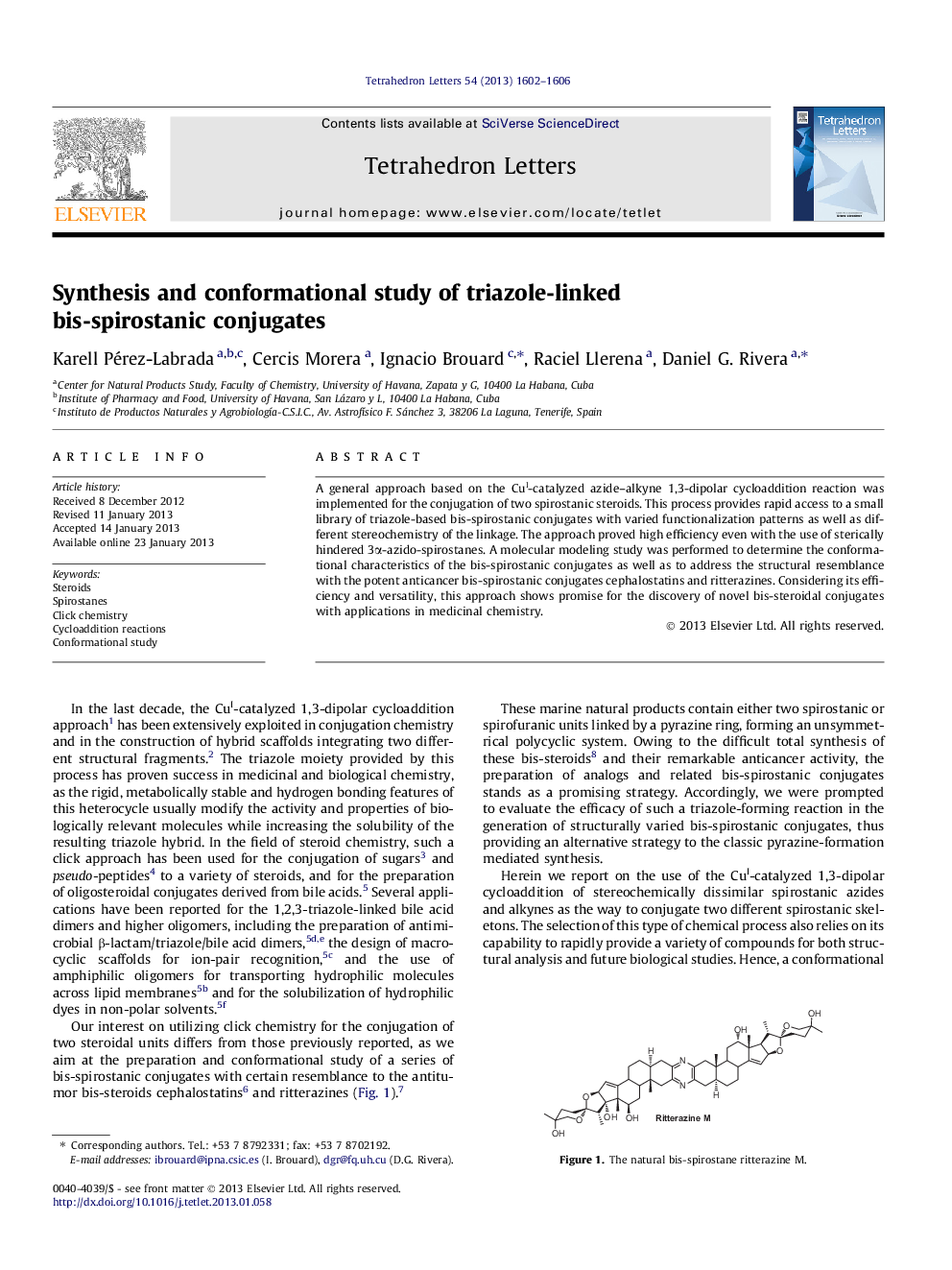
A general approach based on the CuI-catalyzed azide-alkyne 1,3-dipolar cycloaddition reaction was implemented for the conjugation of two spirostanic steroids. This process provides rapid access to a small library of triazole-based bis-spirostanic conjugates with varied functionalization patterns as well as different stereochemistry of the linkage. The approach proved high efficiency even with the use of sterically hindered 3α-azido-spirostanes. A molecular modeling study was performed to determine the conformational characteristics of the bis-spirostanic conjugates as well as to address the structural resemblance with the potent anticancer bis-spirostanic conjugates cephalostatins and ritterazines. Considering its efficiency and versatility, this approach shows promise for the discovery of novel bis-steroidal conjugates with applications in medicinal chemistry.
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry
Authors
Karell Pérez-Labrada, Cercis Morera, Ignacio Brouard, Raciel Llerena, Daniel G. Rivera,