| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5266793 | Tetrahedron Letters | 2012 | 4 Pages |
Abstract
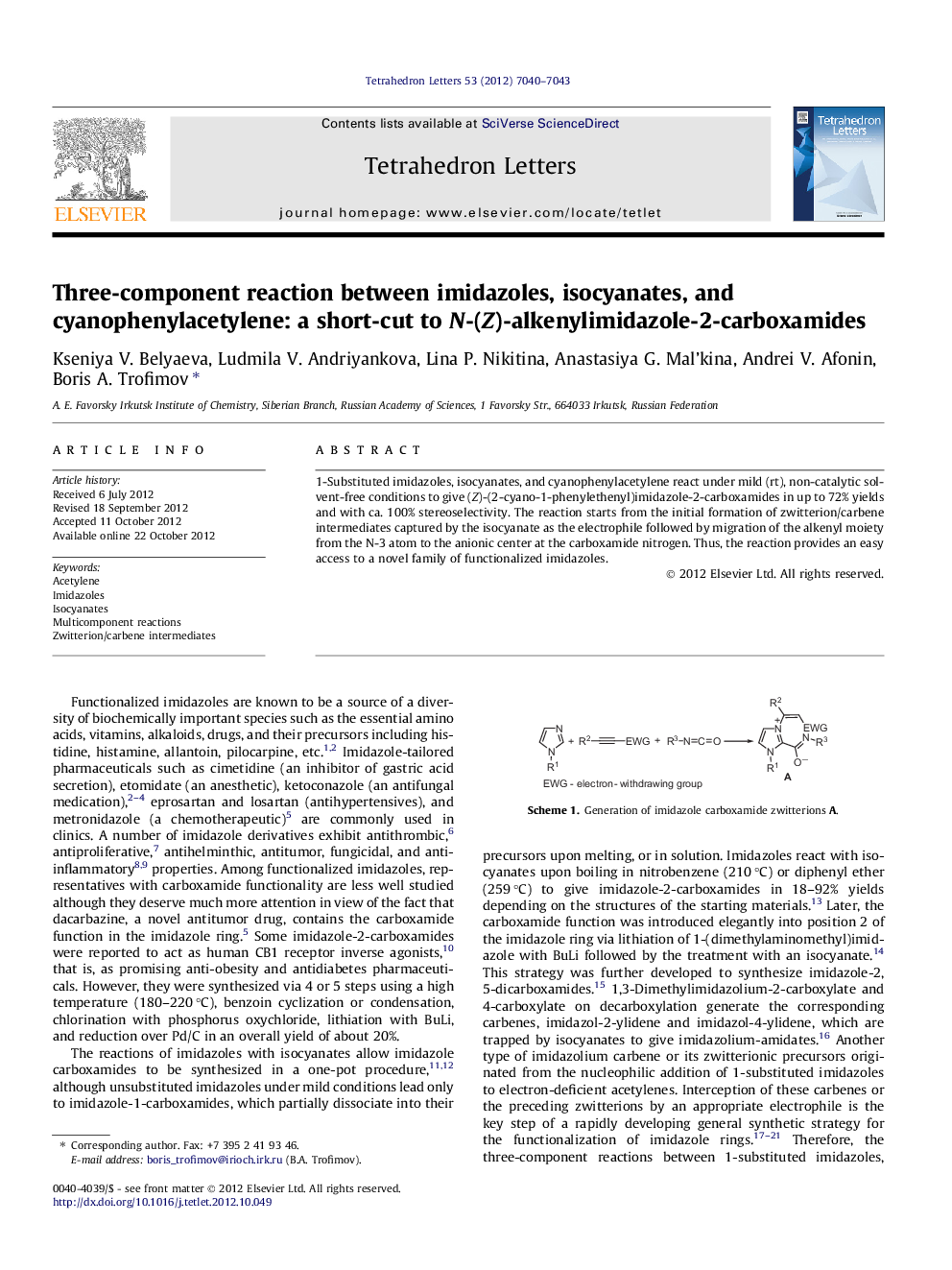
1-Substituted imidazoles, isocyanates, and cyanophenylacetylene react under mild (rt), non-catalytic solvent-free conditions to give (Z)-(2-cyano-1-phenylethenyl)imidazole-2-carboxamides in up to 72% yields and with ca. 100% stereoselectivity. The reaction starts from the initial formation of zwitterion/carbene intermediates captured by the isocyanate as the electrophile followed by migration of the alkenyl moiety from the N-3 atom to the anionic center at the carboxamide nitrogen. Thus, the reaction provides an easy access to a novel family of functionalized imidazoles.
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry
Authors
Kseniya V. Belyaeva, Ludmila V. Andriyankova, Lina P. Nikitina, Anastasiya G. Mal'kina, Andrei V. Afonin, Boris A. Trofimov,