| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5266846 | Tetrahedron Letters | 2011 | 5 Pages |
Abstract
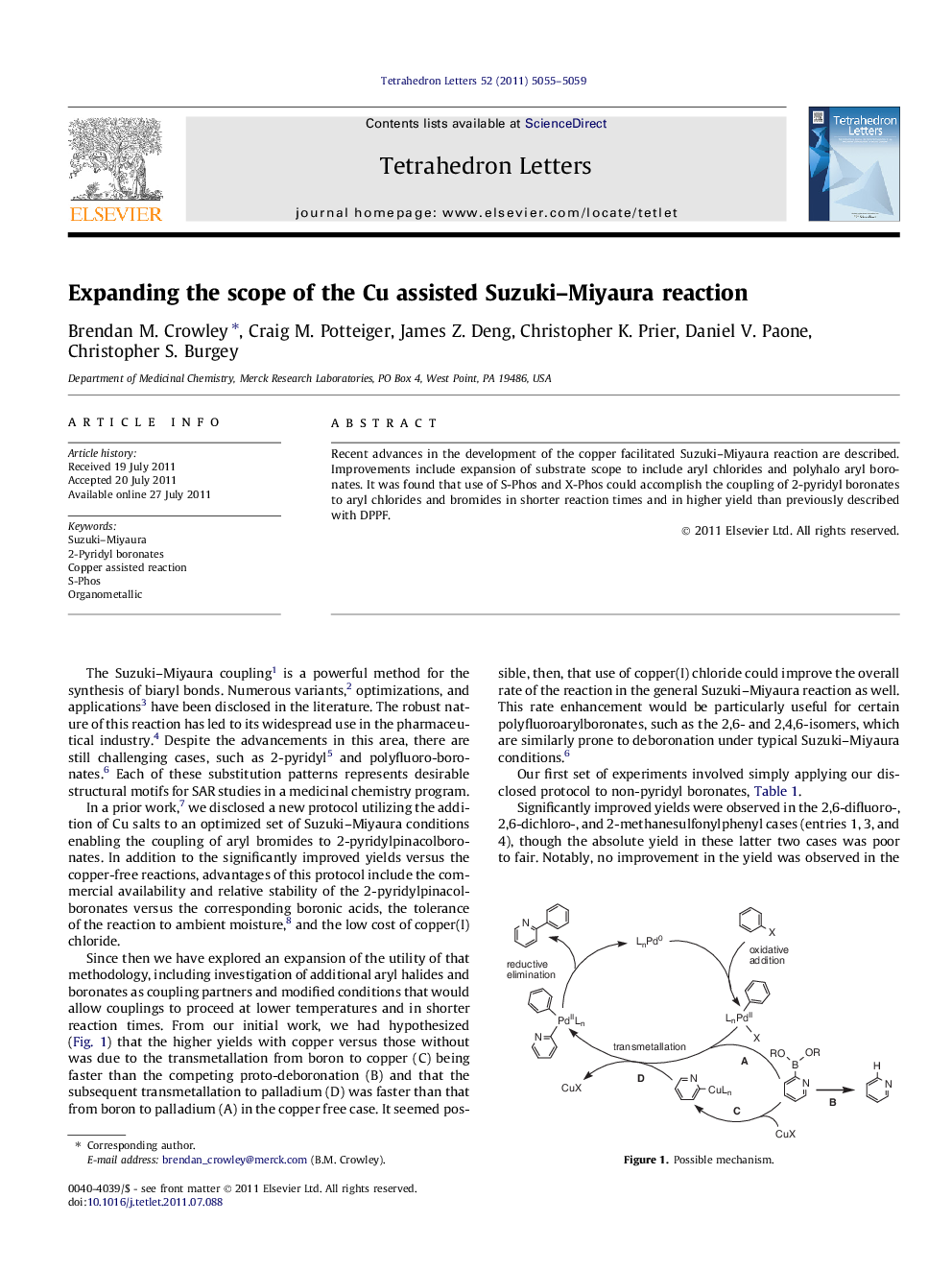
Recent advances in the development of the copper facilitated Suzuki-Miyaura reaction are described. Improvements include expansion of substrate scope to include aryl chlorides and polyhalo aryl boronates. It was found that use of S-Phos and X-Phos could accomplish the coupling of 2-pyridyl boronates to aryl chlorides and bromides in shorter reaction times and in higher yield than previously described with DPPF.
Keywords
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry
Authors
Brendan M. Crowley, Craig M. Potteiger, James Z. Deng, Christopher K. Prier, Daniel V. Paone, Christopher S. Burgey,