| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5267830 | Tetrahedron Letters | 2012 | 4 Pages |
Abstract
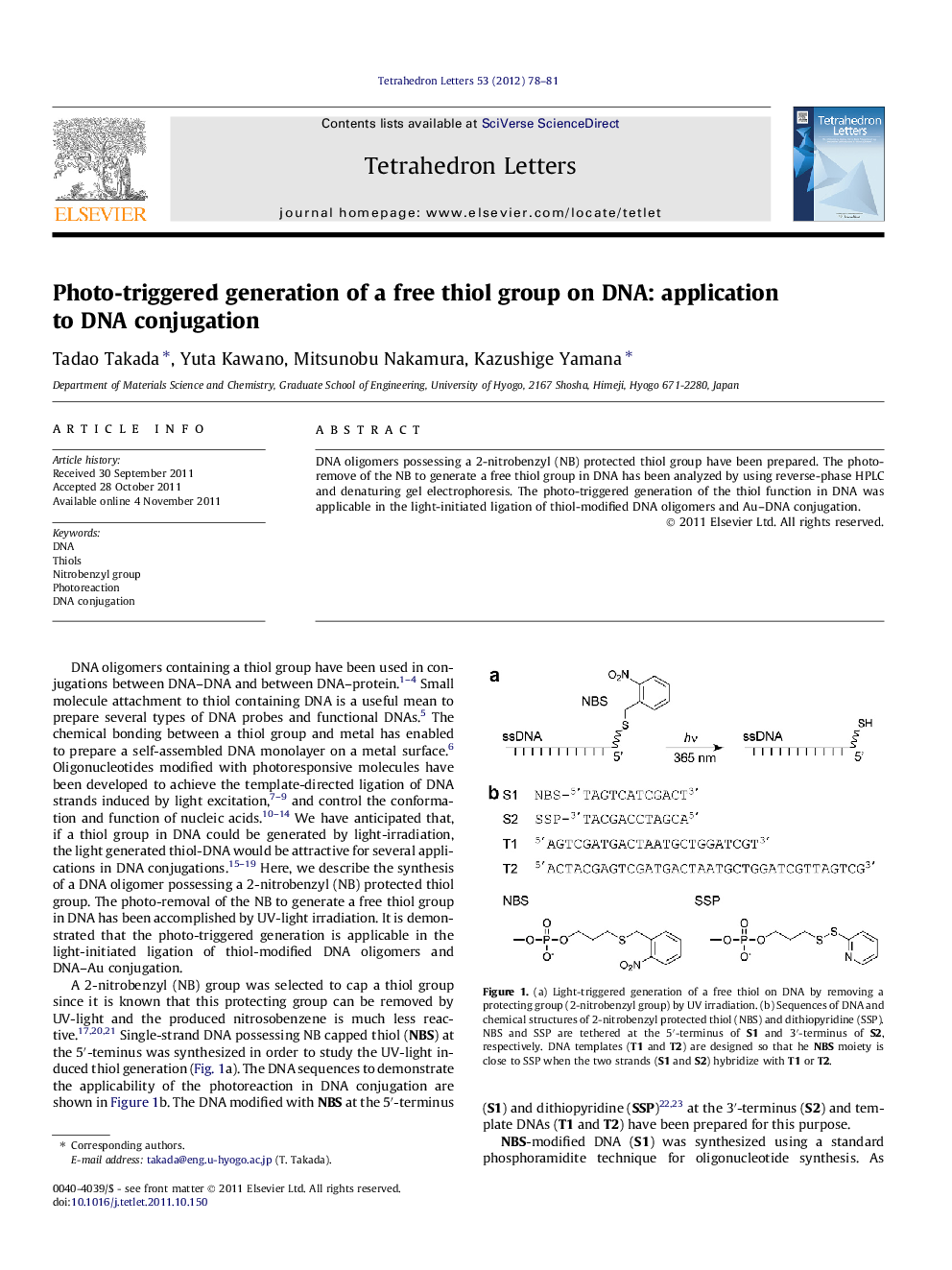
DNA oligomers possessing a 2-nitrobenzyl (NB) protected thiol group have been prepared. The photo-remove of the NB to generate a free thiol group in DNA has been analyzed by using reverse-phase HPLC and denaturing gel electrophoresis. The photo-triggered generation of the thiol function in DNA was applicable in the light-initiated ligation of thiol-modified DNA oligomers and Au-DNA conjugation.
Keywords
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry
Authors
Tadao Takada, Yuta Kawano, Mitsunobu Nakamura, Kazushige Yamana,