| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5272123 | Tetrahedron Letters | 2013 | 4 Pages |
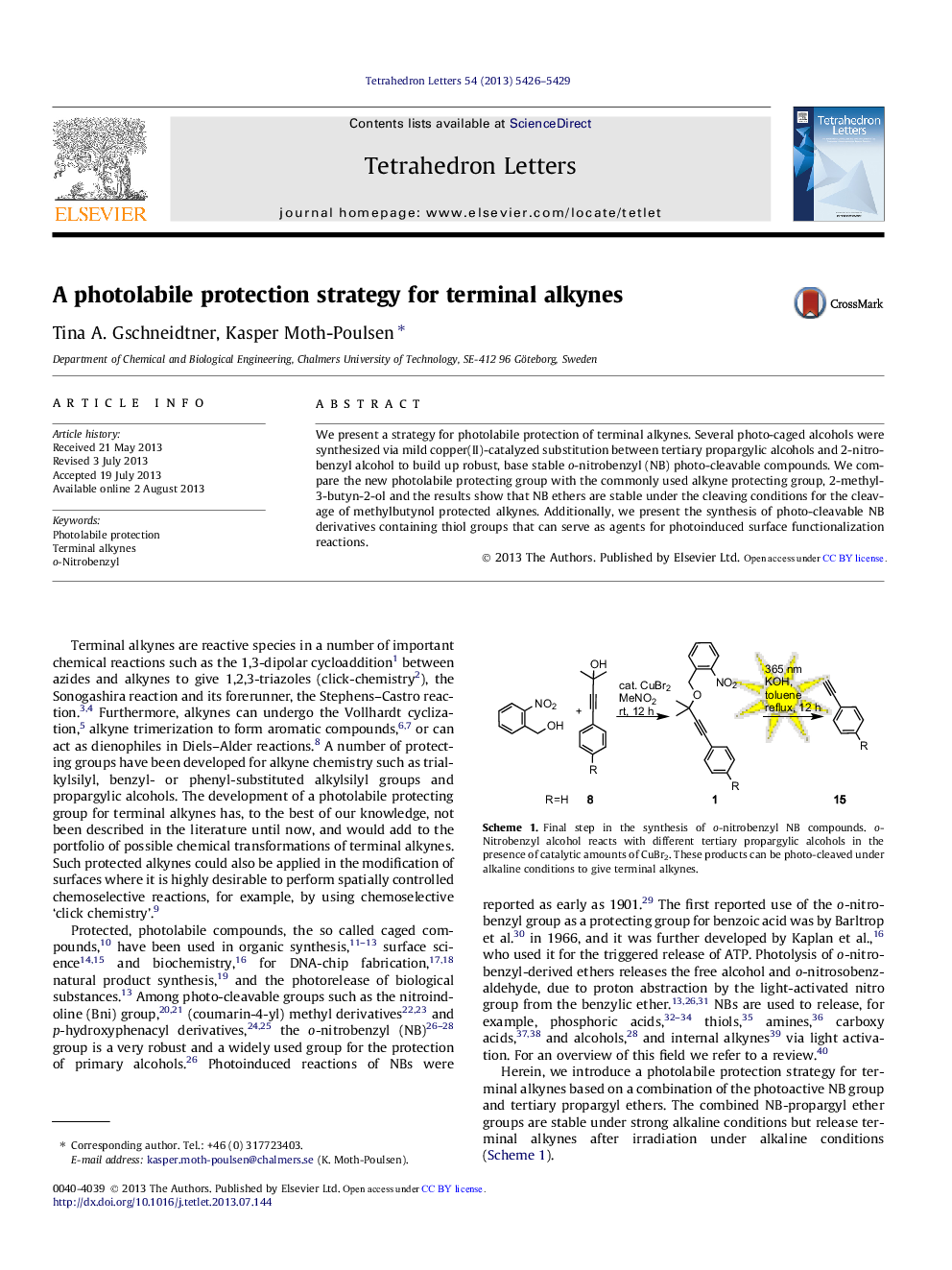
We present a strategy for photolabile protection of terminal alkynes. Several photo-caged alcohols were synthesized via mild copper(II)-catalyzed substitution between tertiary propargylic alcohols and 2-nitrobenzyl alcohol to build up robust, base stable o-nitrobenzyl (NB) photo-cleavable compounds. We compare the new photolabile protecting group with the commonly used alkyne protecting group, 2-methyl-3-butyn-2-ol and the results show that NB ethers are stable under the cleaving conditions for the cleavage of methylbutynol protected alkynes. Additionally, we present the synthesis of photo-cleavable NB derivatives containing thiol groups that can serve as agents for photoinduced surface functionalization reactions.
Graphical abstractDownload full-size image