| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5272128 | Tetrahedron Letters | 2013 | 5 Pages |
Abstract
CuI facilitated three-component reaction of isatin derivatives, l-proline and terminal alkynes containing an amide or ester functional group. The multi-component reaction (MCR) afforded a faster and practical synthesis of spirooxindole derivatives. A range of novel spirooxindoles were synthesized by using this straightforward and one-pot efficient methodology. A representative compound showed significant inhibition of PDE4B enzyme in vitro and good interactions with this protein in silico.
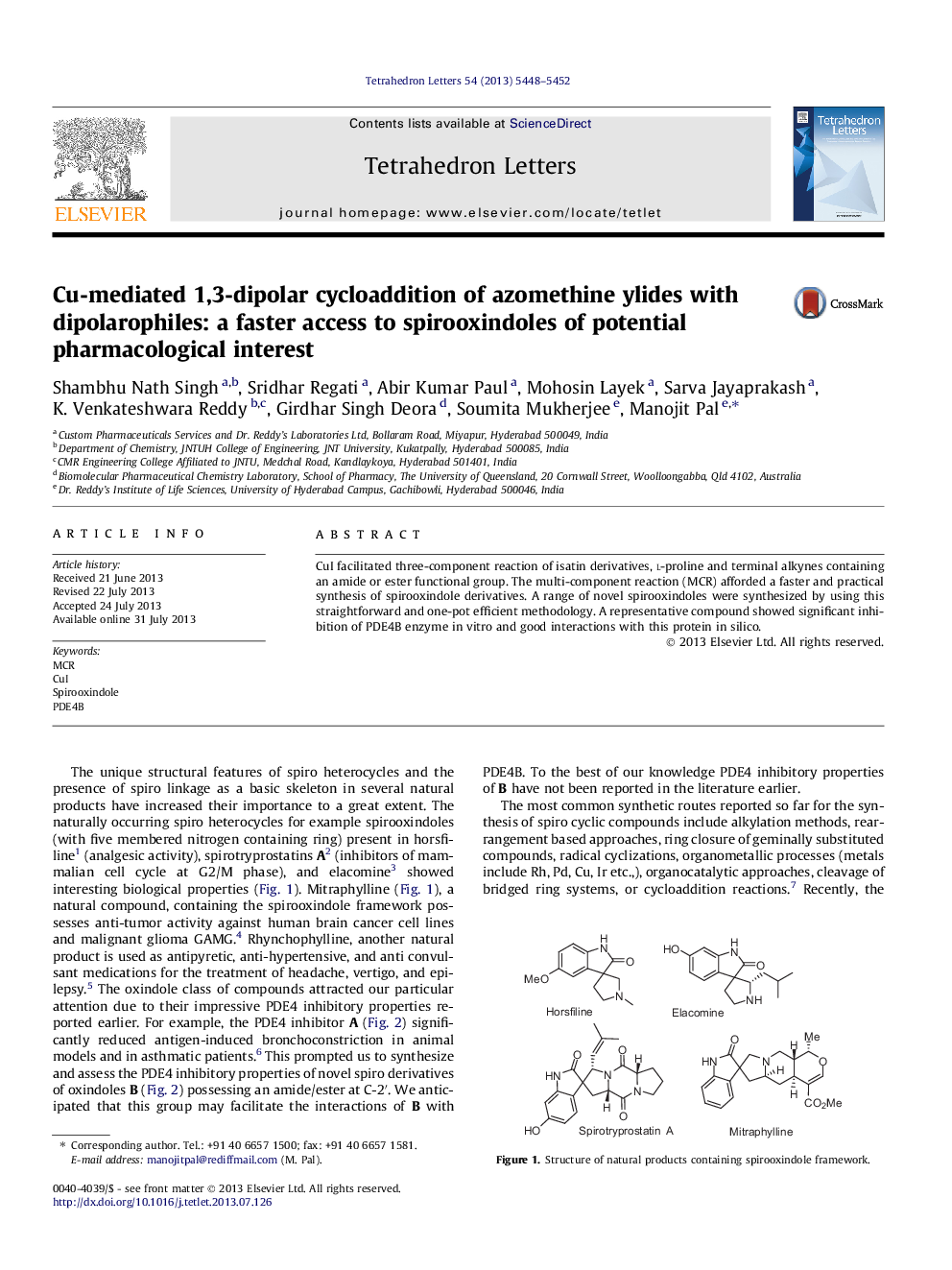
Graphical abstractCu-mediated three-component reaction afforded novel spirooxindoles for the potential inhibition of PDE4.Download full-size image
Keywords
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry
Authors
Shambhu Nath Singh, Sridhar Regati, Abir Kumar Paul, Mohosin Layek, Sarva Jayaprakash, K. Venkateshwara Reddy, Girdhar Singh Deora, Soumita Mukherjee, Manojit Pal,