| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5273521 | Tetrahedron Letters | 2008 | 4 Pages |
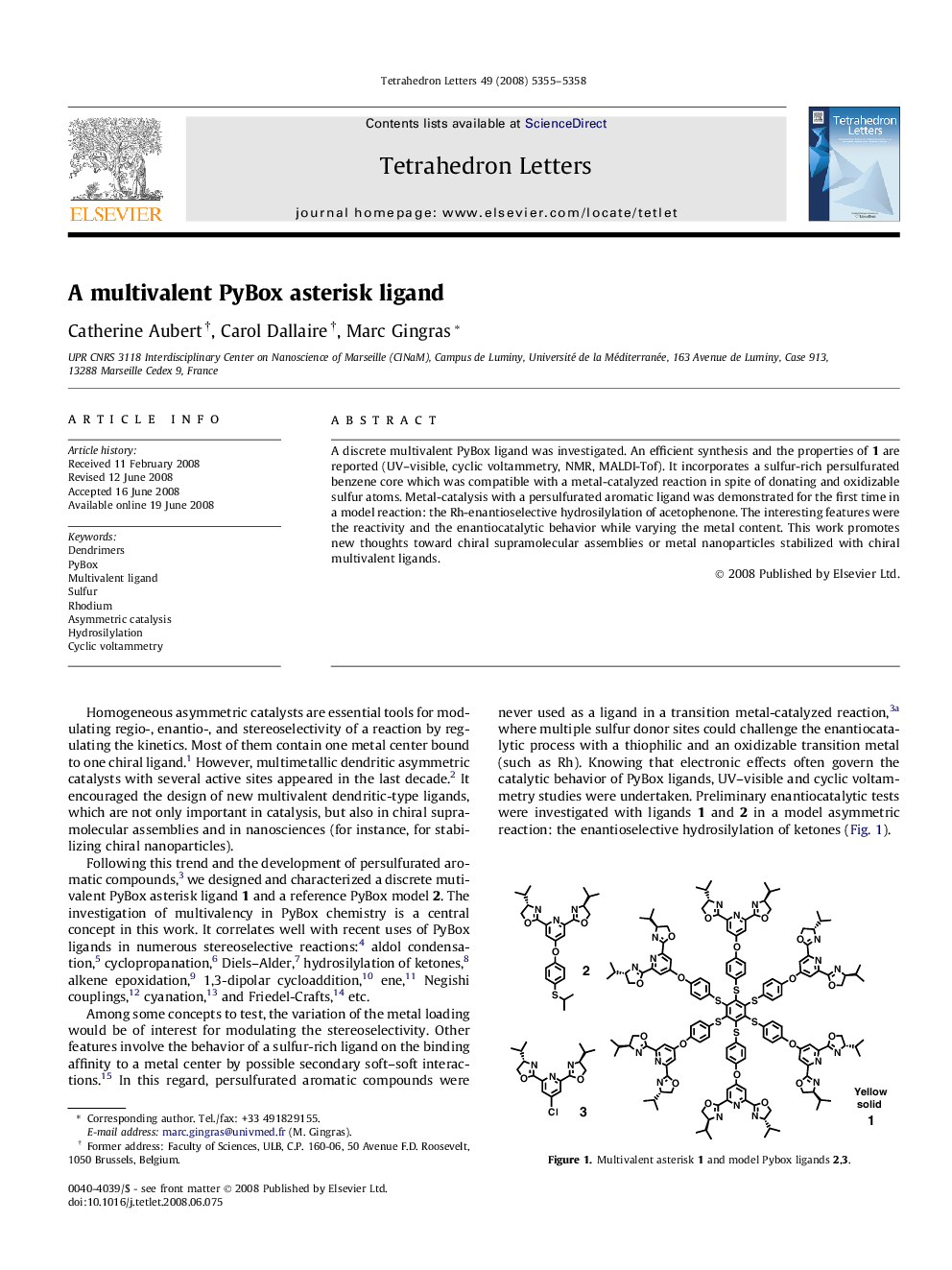
A discrete multivalent PyBox ligand was investigated. An efficient synthesis and the properties of 1 are reported (UV-visible, cyclic voltammetry, NMR, MALDI-Tof). It incorporates a sulfur-rich persulfurated benzene core which was compatible with a metal-catalyzed reaction in spite of donating and oxidizable sulfur atoms. Metal-catalysis with a persulfurated aromatic ligand was demonstrated for the first time in a model reaction: the Rh-enantioselective hydrosilylation of acetophenone. The interesting features were the reactivity and the enantiocatalytic behavior while varying the metal content. This work promotes new thoughts toward chiral supramolecular assemblies or metal nanoparticles stabilized with chiral multivalent ligands.
Graphical abstractThe synthesis, properties (UV-vis, cyclic voltammetry, and MALDI-Tof MS) and multivalency of a sulfur-rich PyBox asterisk ligand were investigated. In spite of multiple coordinating sulfur ligands from a persulfurated benzene core, the asterisk ligand was compatible with a transition metal-catalyzed reaction (an enantioselective Rh-catalyzed hydrosilylation of acetophenone) which was dependent on the metal content. The usefulness of this ligand could be broader for synthesis and it promotes new thoughts toward chiral supramolecular assemblies, metal sensing devices and stabilized metal nanoparticles.Download full-size image