| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5273983 | Tetrahedron Letters | 2013 | 5 Pages |
Abstract
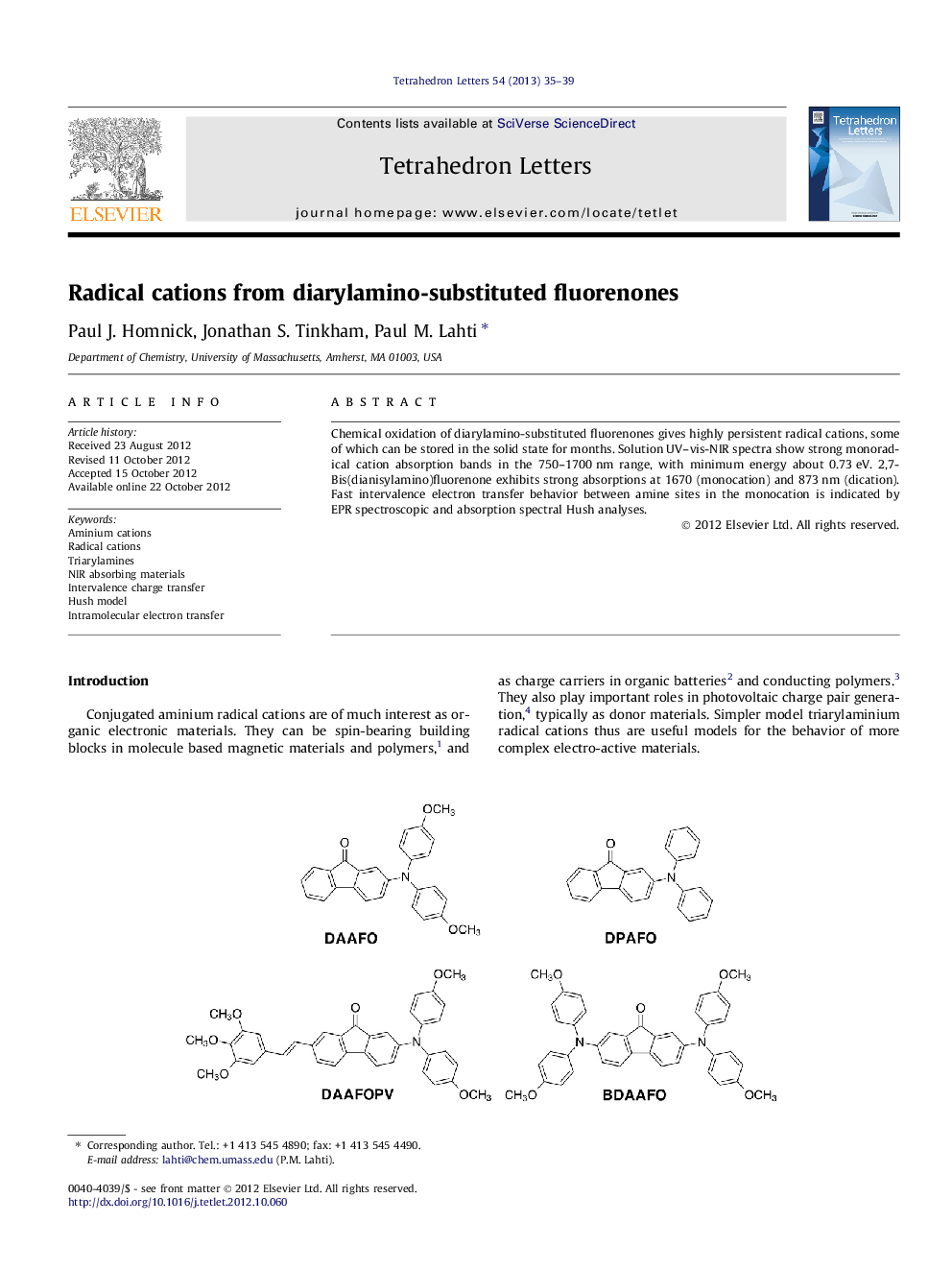
Chemical oxidation of diarylamino-substituted fluorenones gives highly persistent radical cations, some of which can be stored in the solid state for months. Solution UV-vis-NIR spectra show strong monoradical cation absorption bands in the 750-1700Â nm range, with minimum energy about 0.73Â eV. 2,7-Bis(dianisylamino)fluorenone exhibits strong absorptions at 1670 (monocation) and 873Â nm (dication). Fast intervalence electron transfer behavior between amine sites in the monocation is indicated by EPR spectroscopic and absorption spectral Hush analyses.
Graphical abstractDownload full-size image
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry
Authors
Paul J. Homnick, Jonathan S. Tinkham, Paul M. Lahti,