| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5274584 | Tetrahedron Letters | 2009 | 6 Pages |
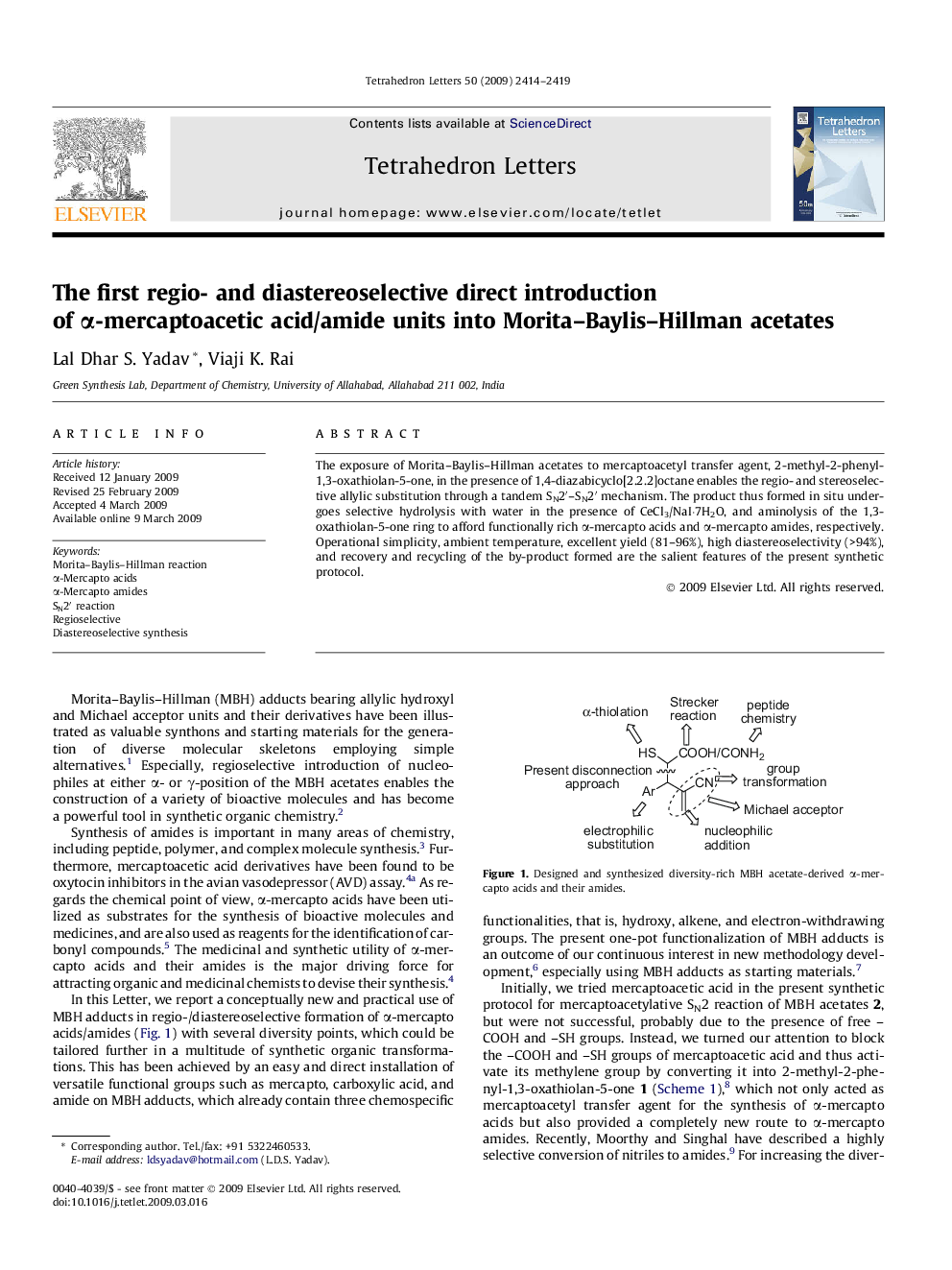
The exposure of Morita-Baylis-Hillman acetates to mercaptoacetyl transfer agent, 2-methyl-2-phenyl-1,3-oxathiolan-5-one, in the presence of 1,4-diazabicyclo[2.2.2]octane enables the regio- and stereoselective allylic substitution through a tandem SN2â²-SN2â² mechanism. The product thus formed in situ undergoes selective hydrolysis with water in the presence of CeCl3/NaI·7H2O, and aminolysis of the 1,3-oxathiolan-5-one ring to afford functionally rich α-mercapto acids and α-mercapto amides, respectively. Operational simplicity, ambient temperature, excellent yield (81-96%), high diastereoselectivity (>94%), and recovery and recycling of the by-product formed are the salient features of the present synthetic protocol.
Graphical abstractDownload full-size image