| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5274945 | Tetrahedron Letters | 2007 | 4 Pages |
Abstract
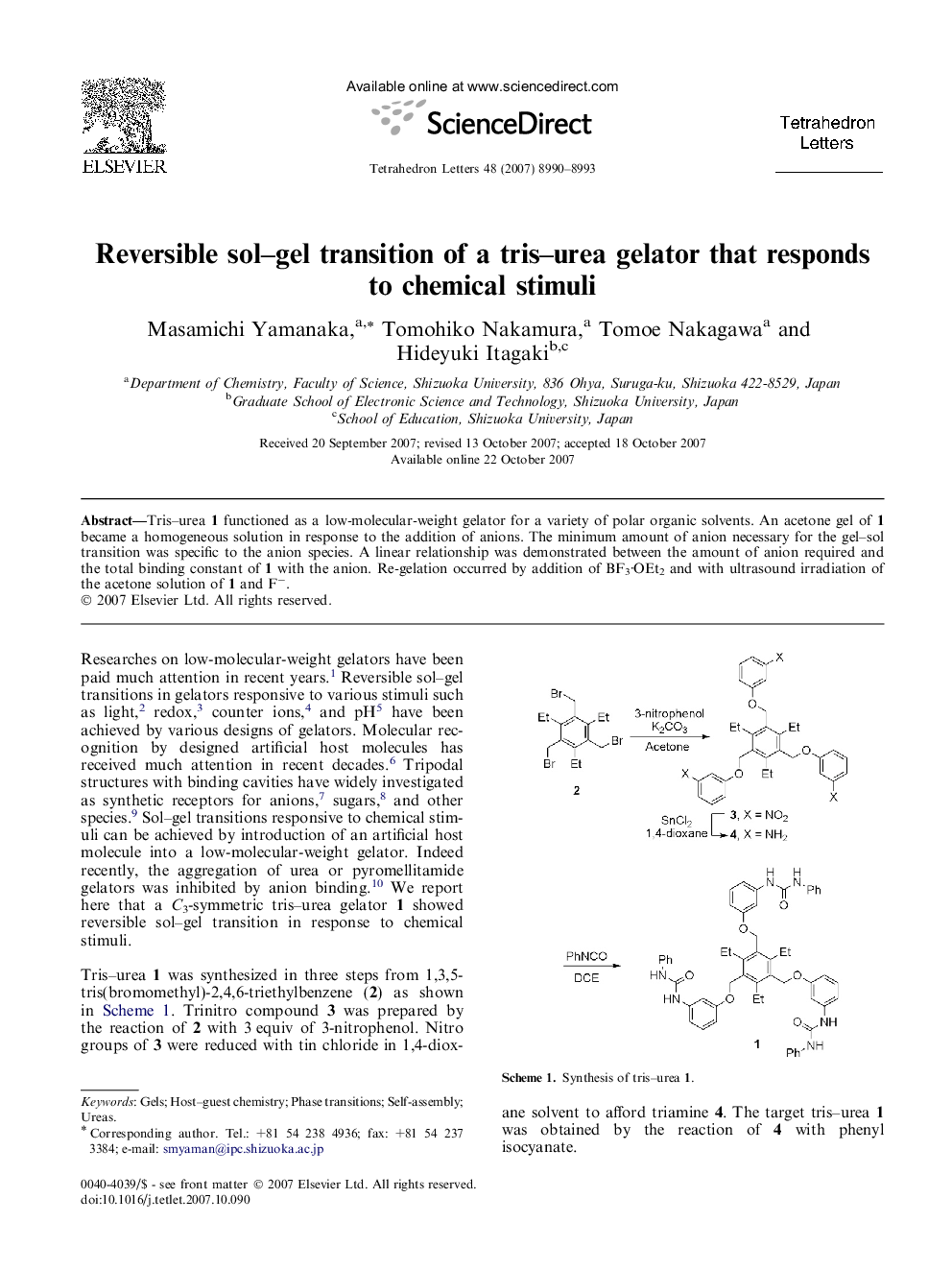
Tris-urea 1 functioned as a low-molecular-weight gelator for a variety of polar organic solvents. An acetone gel of 1 became a homogeneous solution in response to the addition of anions. The minimum amount of anion necessary for the gel-sol transition was specific to the anion species. A linear relationship was demonstrated between the amount of anion required and the total binding constant of 1 with the anion. Re-gelation occurred by addition of BF3·OEt2 and with ultrasound irradiation of the acetone solution of 1 and Fâ.
Graphical abstractDownload full-size image
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry
Authors
Masamichi Yamanaka, Tomohiko Nakamura, Tomoe Nakagawa, Hideyuki Itagaki,