| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5276644 | Tetrahedron Letters | 2007 | 4 Pages |
Abstract
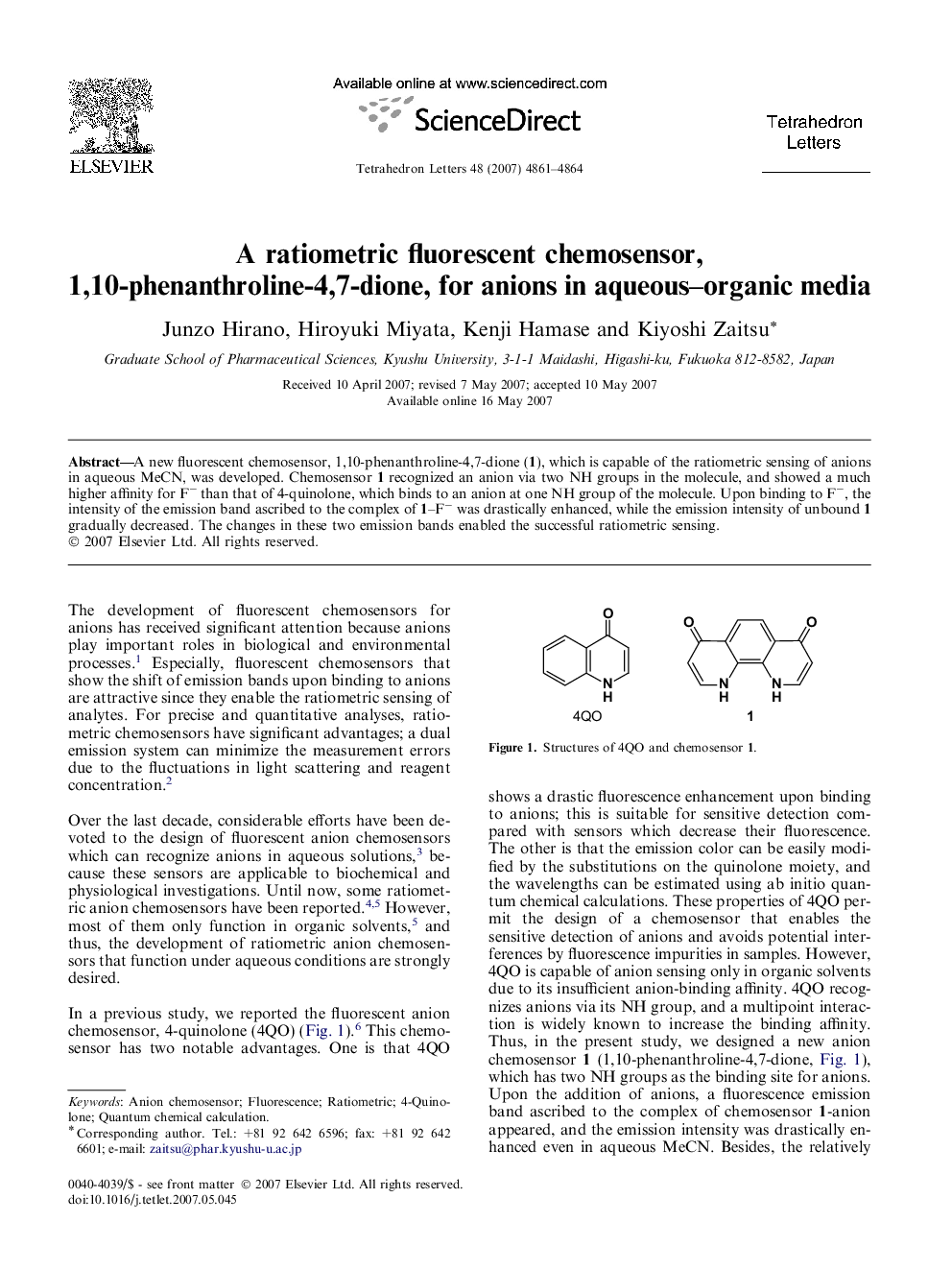
A new fluorescent chemosensor, 1,10-phenanthroline-4,7-dione (1), which is capable of the ratiometric sensing of anions in aqueous MeCN, was developed. Chemosensor 1 recognized an anion via two NH groups in the molecule, and showed a much higher affinity for Fâ than that of 4-quinolone, which binds to an anion at one NH group of the molecule. Upon binding to Fâ, the intensity of the emission band ascribed to the complex of 1-Fâ was drastically enhanced, while the emission intensity of unbound 1 gradually decreased. The changes in these two emission bands enabled the successful ratiometric sensing.
Graphical abstractDownload full-size image
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry
Authors
Junzo Hirano, Hiroyuki Miyata, Kenji Hamase, Kiyoshi Zaitsu,