| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5277519 | Tetrahedron Letters | 2007 | 5 Pages |
Abstract
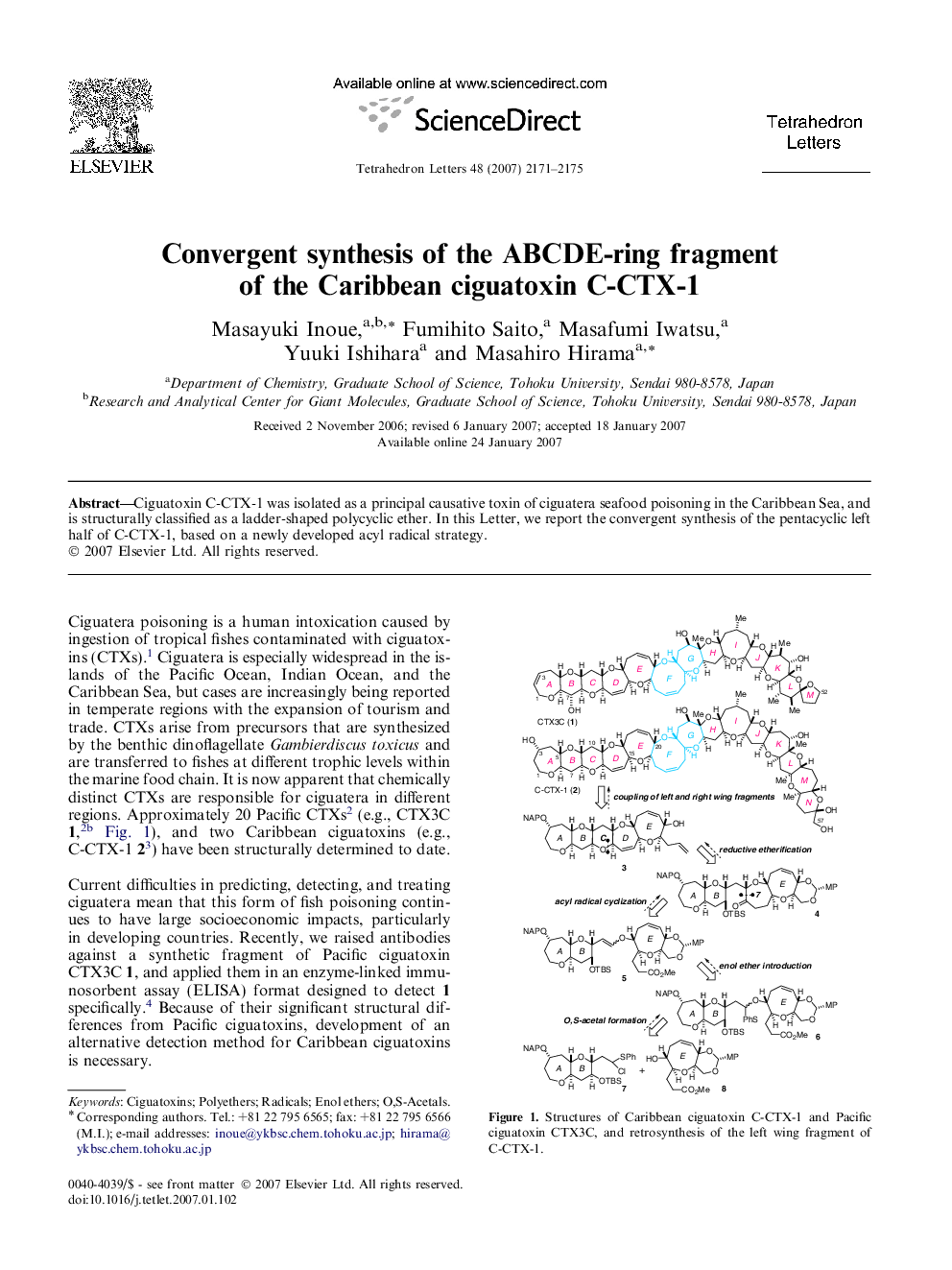
Ciguatoxin C-CTX-1 was isolated as a principal causative toxin of ciguatera seafood poisoning in the Caribbean Sea, and is structurally classified as a ladder-shaped polycyclic ether. In this Letter, we report the convergent synthesis of the pentacyclic left half of C-CTX-1, based on a newly developed acyl radical strategy.
Graphical abstractDownload full-size image
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry
Authors
Masayuki Inoue, Fumihito Saito, Masafumi Iwatsu, Yuuki Ishihara, Masahiro Hirama,