| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5277695 | Tetrahedron Letters | 2011 | 5 Pages |
Abstract
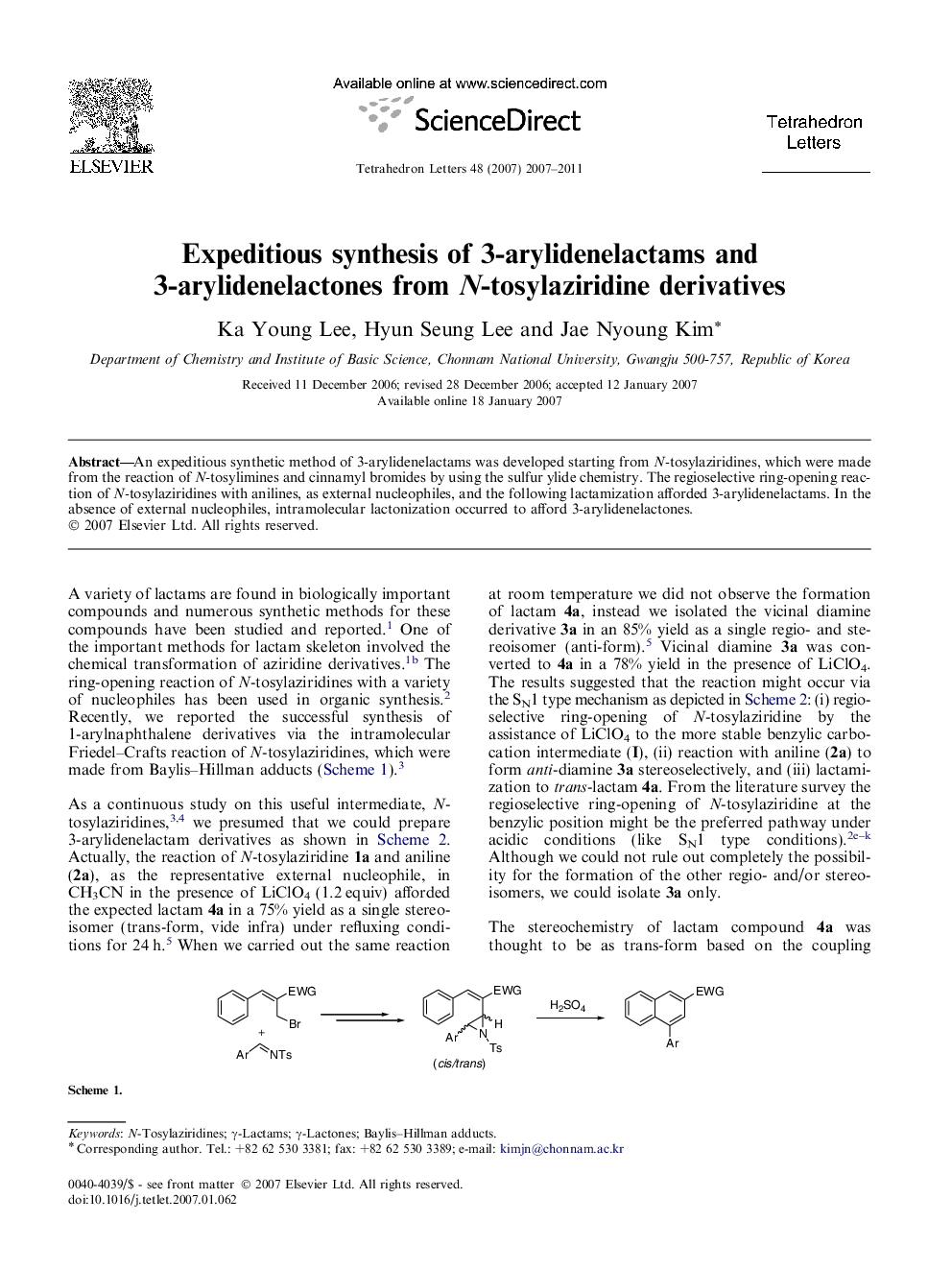
An expeditious synthetic method of 3-arylidenelactams was developed starting from N-tosylaziridines, which were made from the reaction of N-tosylimines and cinnamyl bromides by using the sulfur ylide chemistry. The regioselective ring-opening reaction of N-tosylaziridines with anilines, as external nucleophiles, and the following lactamization afforded 3-arylidenelactams. In the absence of external nucleophiles, intramolecular lactonization occurred to afford 3-arylidenelactones.
Graphical abstractDownload full-size image
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry
Authors
Ka Young Lee, Hyun Seung Lee, Jae Nyoung Kim,