| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5278133 | Tetrahedron Letters | 2006 | 4 Pages |
Abstract
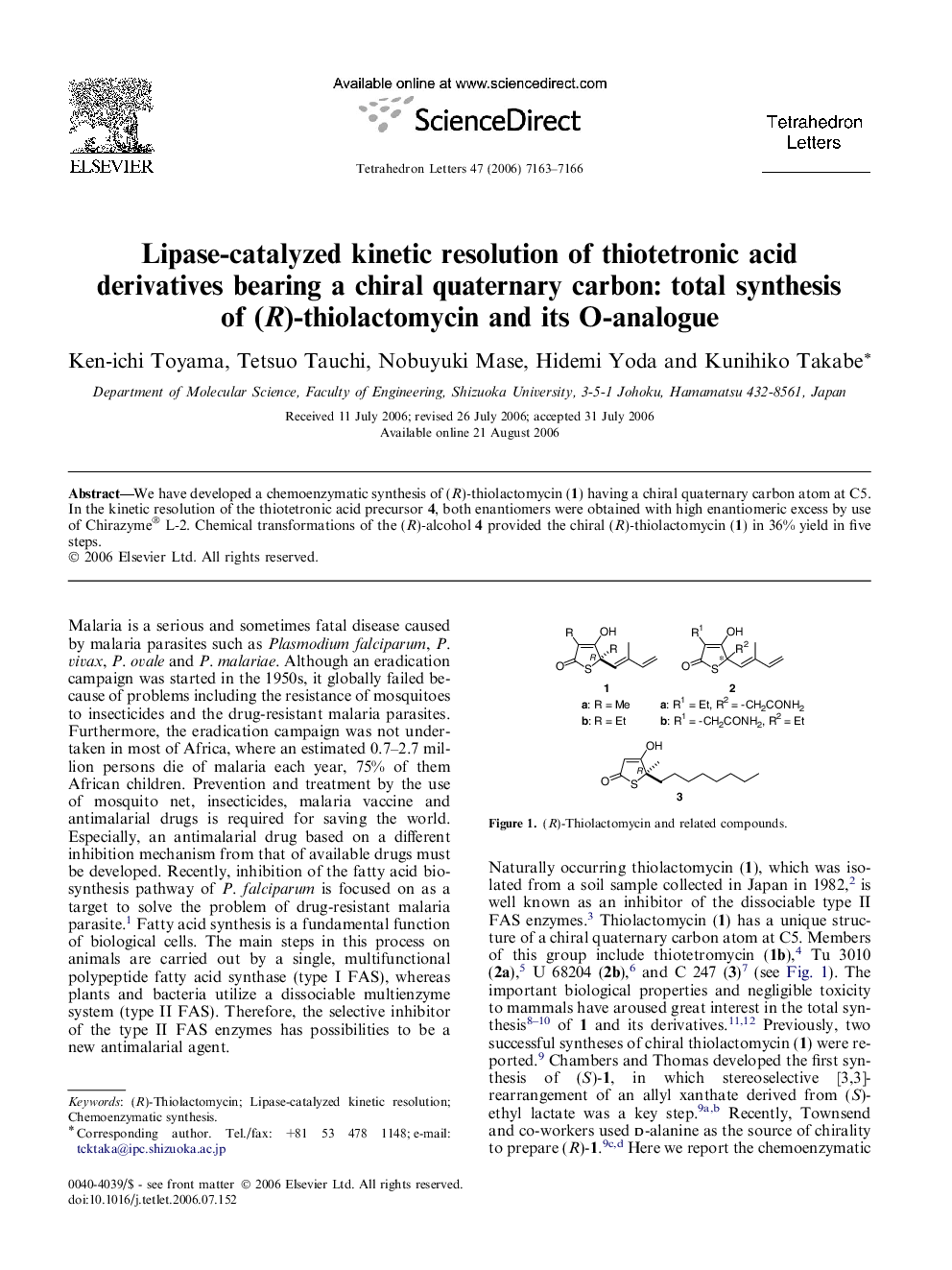
We have developed a chemoenzymatic synthesis of (R)-thiolactomycin (1) having a chiral quaternary carbon atom at C5. In the kinetic resolution of the thiotetronic acid precursor 4, both enantiomers were obtained with high enantiomeric excess by use of Chirazyme® L-2. Chemical transformations of the (R)-alcohol 4 provided the chiral (R)-thiolactomycin (1) in 36% yield in five steps.
Graphical abstractLipase-catalyzed kinetic resolution of thiotetronic acid derivatives having a quaternary carbon was investigated. Total synthesis of (R)-thiolactomycin from (R)-alcohol was achieved in 36% yield in five steps.Download full-size image
Keywords
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry
Authors
Ken-ichi Toyama, Tetsuo Tauchi, Nobuyuki Mase, Hidemi Yoda, Kunihiko Takabe,