| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5279530 | Tetrahedron Letters | 2005 | 4 Pages |
Abstract
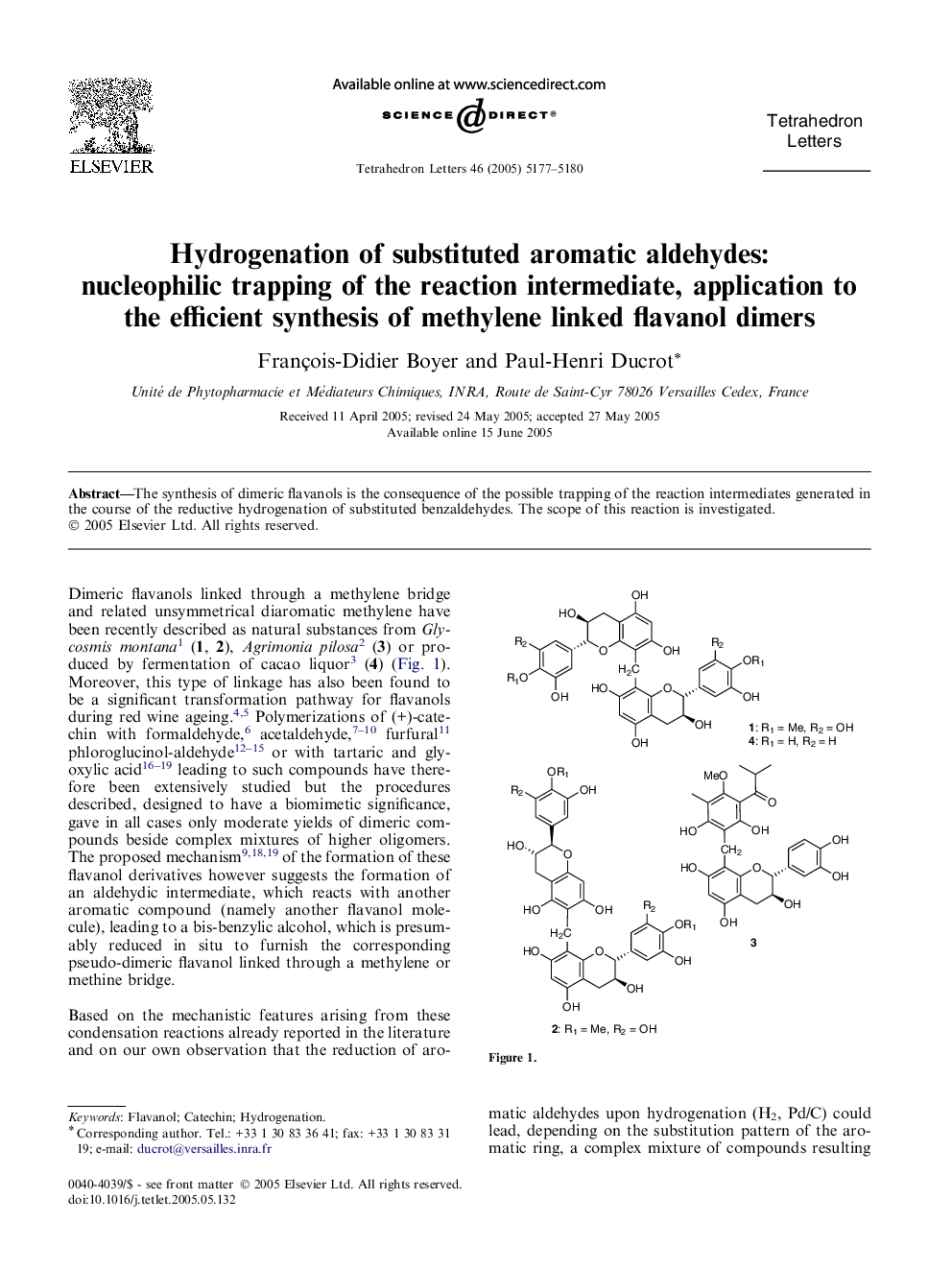
The synthesis of dimeric flavanols is the consequence of the possible trapping of the reaction intermediates generated in the course of the reductive hydrogenation of substituted benzaldehydes. The scope of this reaction is investigated.
Graphical abstractMethylene linked flavanol dimers and analogues have been synthesized through hydrogenation of the corresponding 8-formyl flavanol in the presence of an appropriate nucleophile.Download full-size image
Keywords
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry
Authors
François-Didier Boyer, Paul-Henri Ducrot,