| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5279580 | Tetrahedron Letters | 2006 | 4 Pages |
Abstract
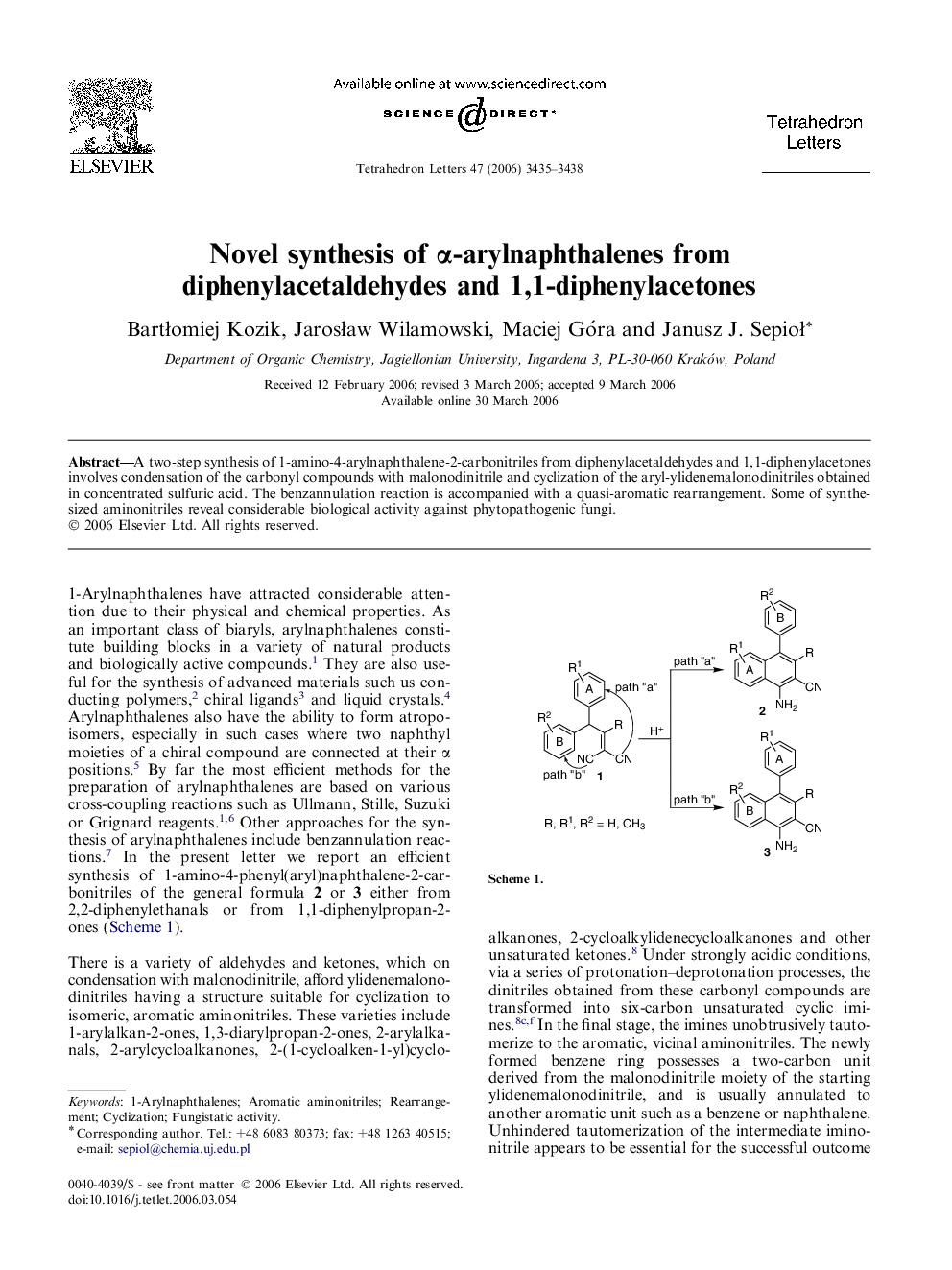
A two-step synthesis of 1-amino-4-arylnaphthalene-2-carbonitriles from diphenylacetaldehydes and 1,1-diphenylacetones involves condensation of the carbonyl compounds with malonodinitrile and cyclization of the aryl-ylidenemalonodinitriles obtained in concentrated sulfuric acid. The benzannulation reaction is accompanied with a quasi-aromatic rearrangement. Some of synthesized aminonitriles reveal considerable biological activity against phytopathogenic fungi.
Graphical abstractDownload full-size image
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry
Authors
BartÅomiej Kozik, JarosÅaw Wilamowski, Maciej Góra, Janusz J. SepioÅ,