| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5279655 | Tetrahedron Letters | 2010 | 4 Pages |
Abstract
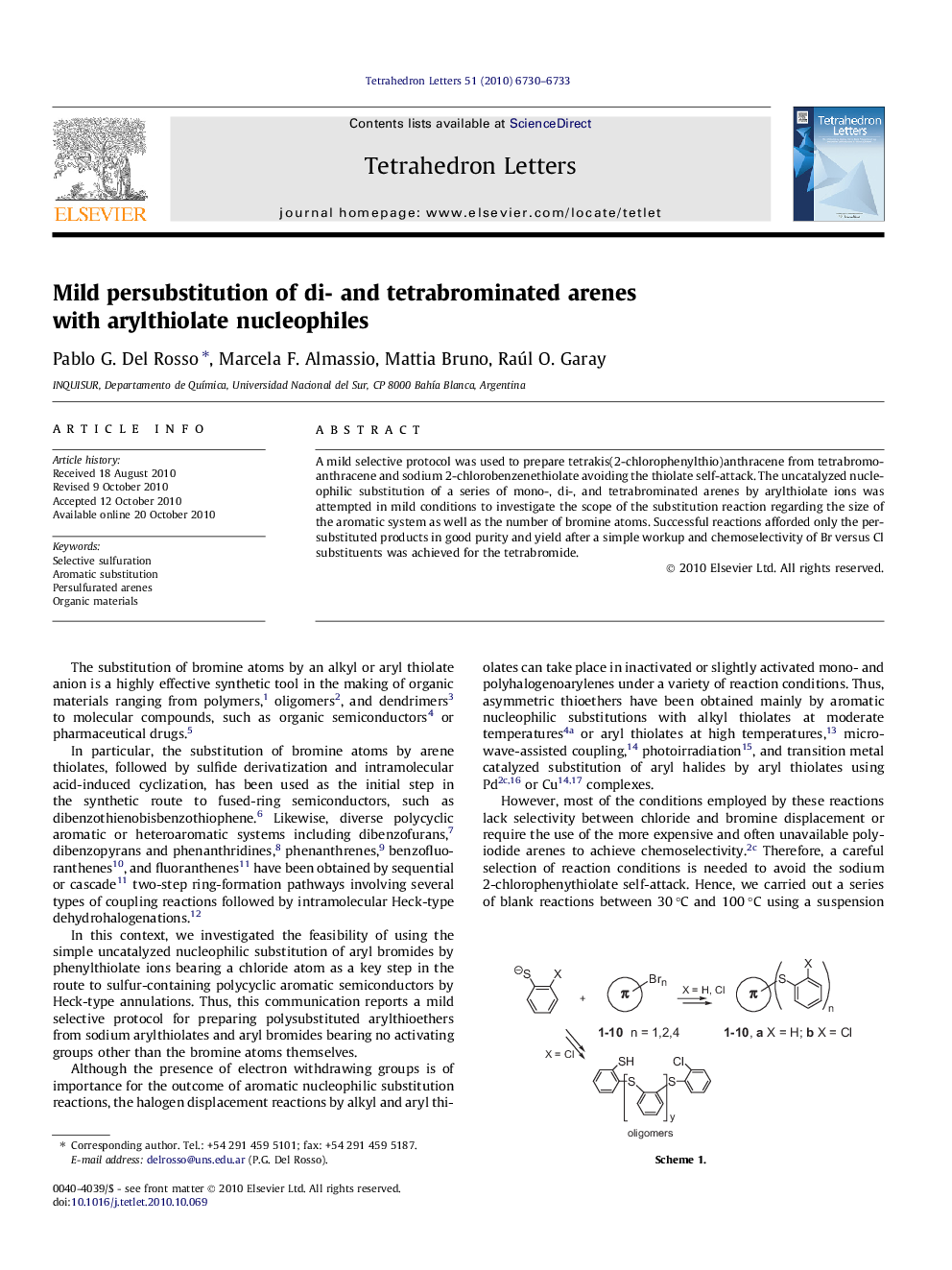
A mild selective protocol was used to prepare tetrakis(2-chlorophenylthio)anthracene from tetrabromoanthracene and sodium 2-chlorobenzenethiolate avoiding the thiolate self-attack. The uncatalyzed nucleophilic substitution of a series of mono-, di-, and tetrabrominated arenes by arylthiolate ions was attempted in mild conditions to investigate the scope of the substitution reaction regarding the size of the aromatic system as well as the number of bromine atoms. Successful reactions afforded only the persubstituted products in good purity and yield after a simple workup and chemoselectivity of Br versus Cl substituents was achieved for the tetrabromide.
Graphical abstractDownload full-size image
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry
Authors
Pablo G. Del Rosso, Marcela F. Almassio, Mattia Bruno, Raúl O. Garay,