| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5280795 | Tetrahedron Letters | 2007 | 4 Pages |
Abstract
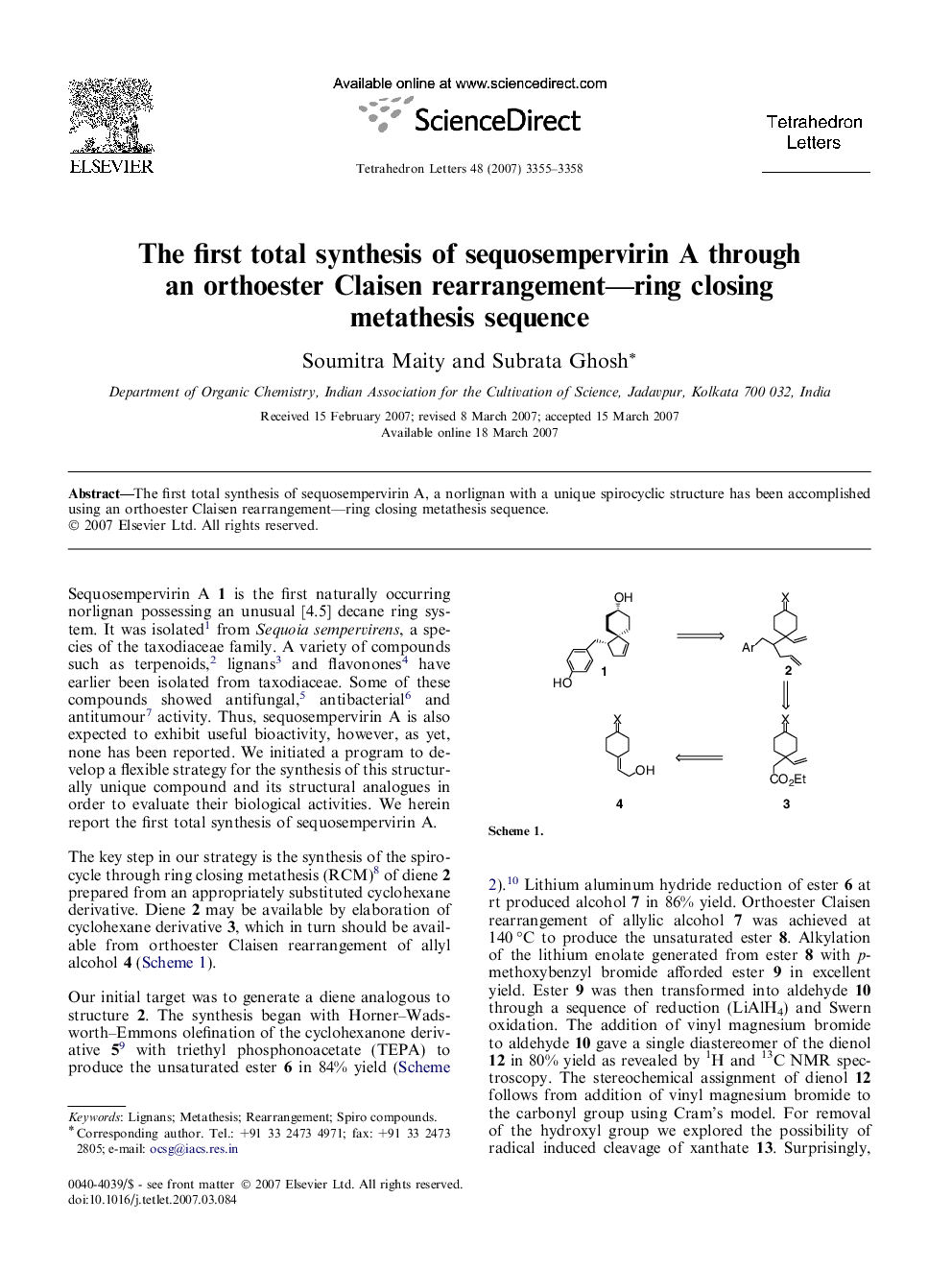
The first total synthesis of sequosempervirin A, a norlignan with a unique spirocyclic structure has been accomplished using an orthoester Claisen rearrangement-ring closing metathesis sequence.
Graphical abstractThe first total synthesis of sequosempervirin A, a norlignan with a unique spirocyclic structure is described.Download full-size image
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry
Authors
Soumitra Maity, Subrata Ghosh,