| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5281041 | Tetrahedron Letters | 2010 | 4 Pages |
Abstract
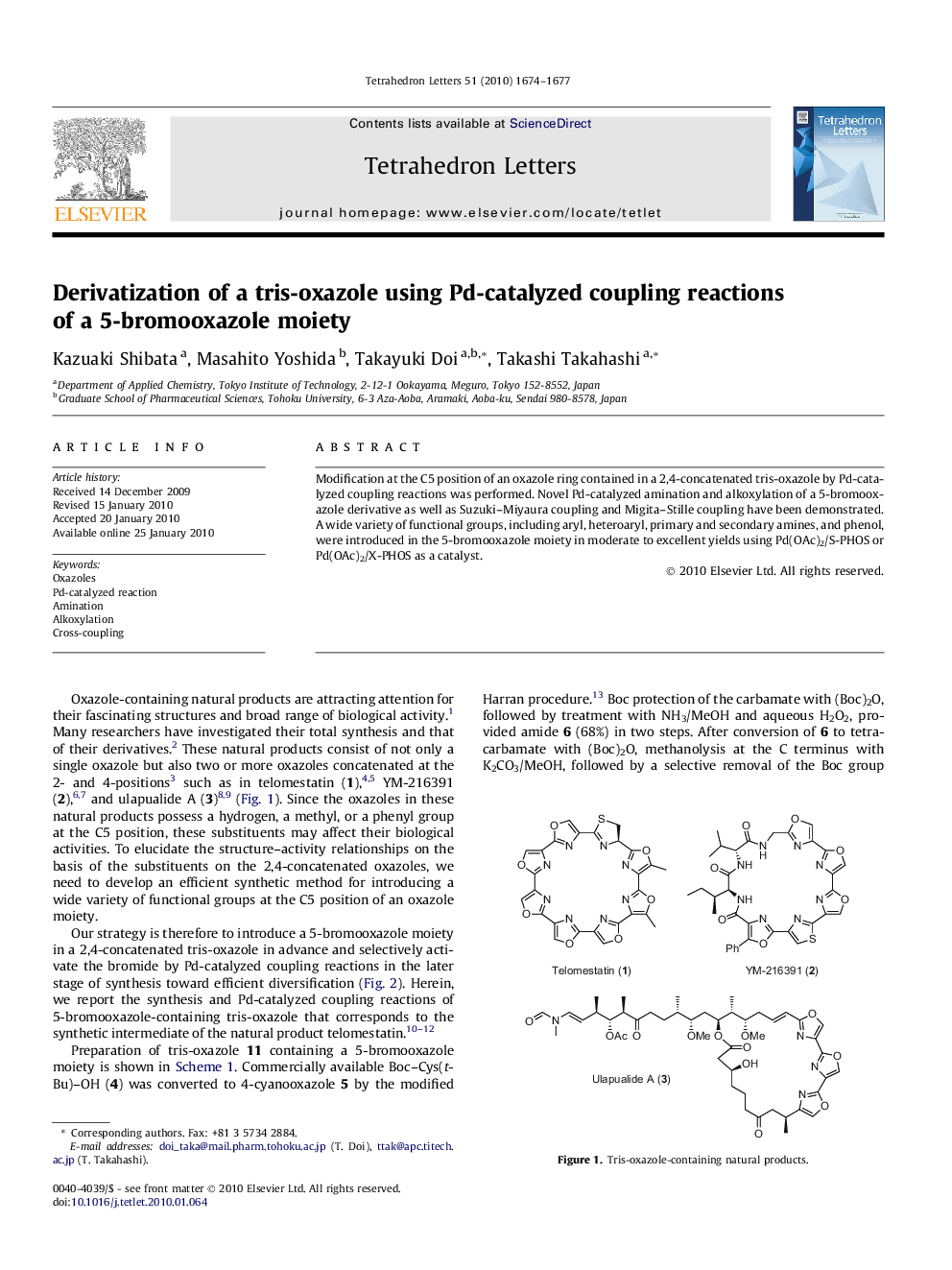
Modification at the C5 position of an oxazole ring contained in a 2,4-concatenated tris-oxazole by Pd-catalyzed coupling reactions was performed. Novel Pd-catalyzed amination and alkoxylation of a 5-bromooxazole derivative as well as Suzuki-Miyaura coupling and Migita-Stille coupling have been demonstrated. A wide variety of functional groups, including aryl, heteroaryl, primary and secondary amines, and phenol, were introduced in the 5-bromooxazole moiety in moderate to excellent yields using Pd(OAc)2/S-PHOS or Pd(OAc)2/X-PHOS as a catalyst.
Graphical abstractDownload full-size image
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry
Authors
Kazuaki Shibata, Masahito Yoshida, Takayuki Doi, Takashi Takahashi,