| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5283330 | Tetrahedron Letters | 2008 | 4 Pages |
Abstract
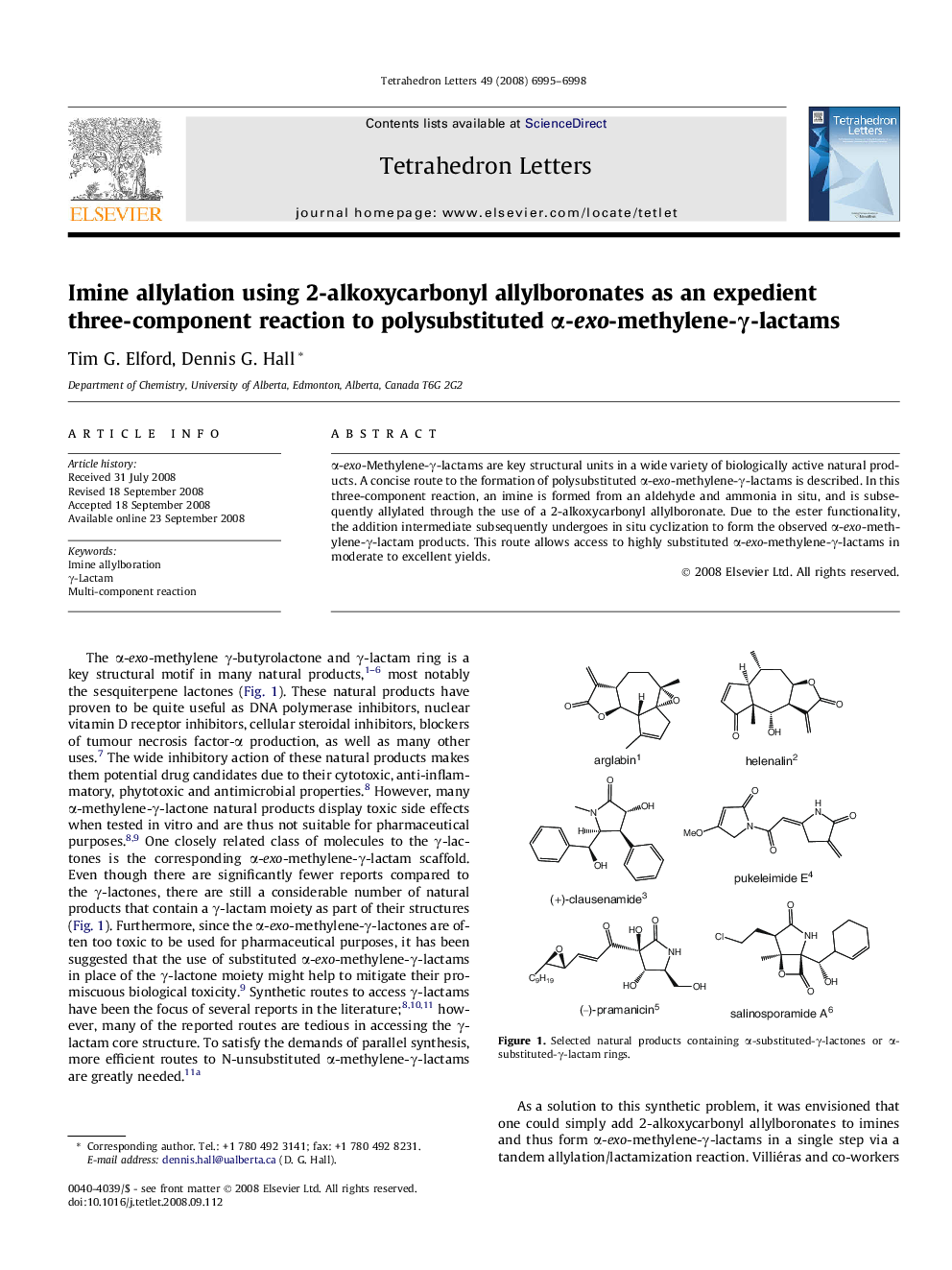
α-exo-Methylene-γ-lactams are key structural units in a wide variety of biologically active natural products. A concise route to the formation of polysubstituted α-exo-methylene-γ-lactams is described. In this three-component reaction, an imine is formed from an aldehyde and ammonia in situ, and is subsequently allylated through the use of a 2-alkoxycarbonyl allylboronate. Due to the ester functionality, the addition intermediate subsequently undergoes in situ cyclization to form the observed α-exo-methylene-γ-lactam products. This route allows access to highly substituted α-exo-methylene-γ-lactams in moderate to excellent yields.
Keywords
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry
Authors
Tim G. Elford, Dennis G. Hall,