| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5283663 | Tetrahedron Letters | 2007 | 4 Pages |
Abstract
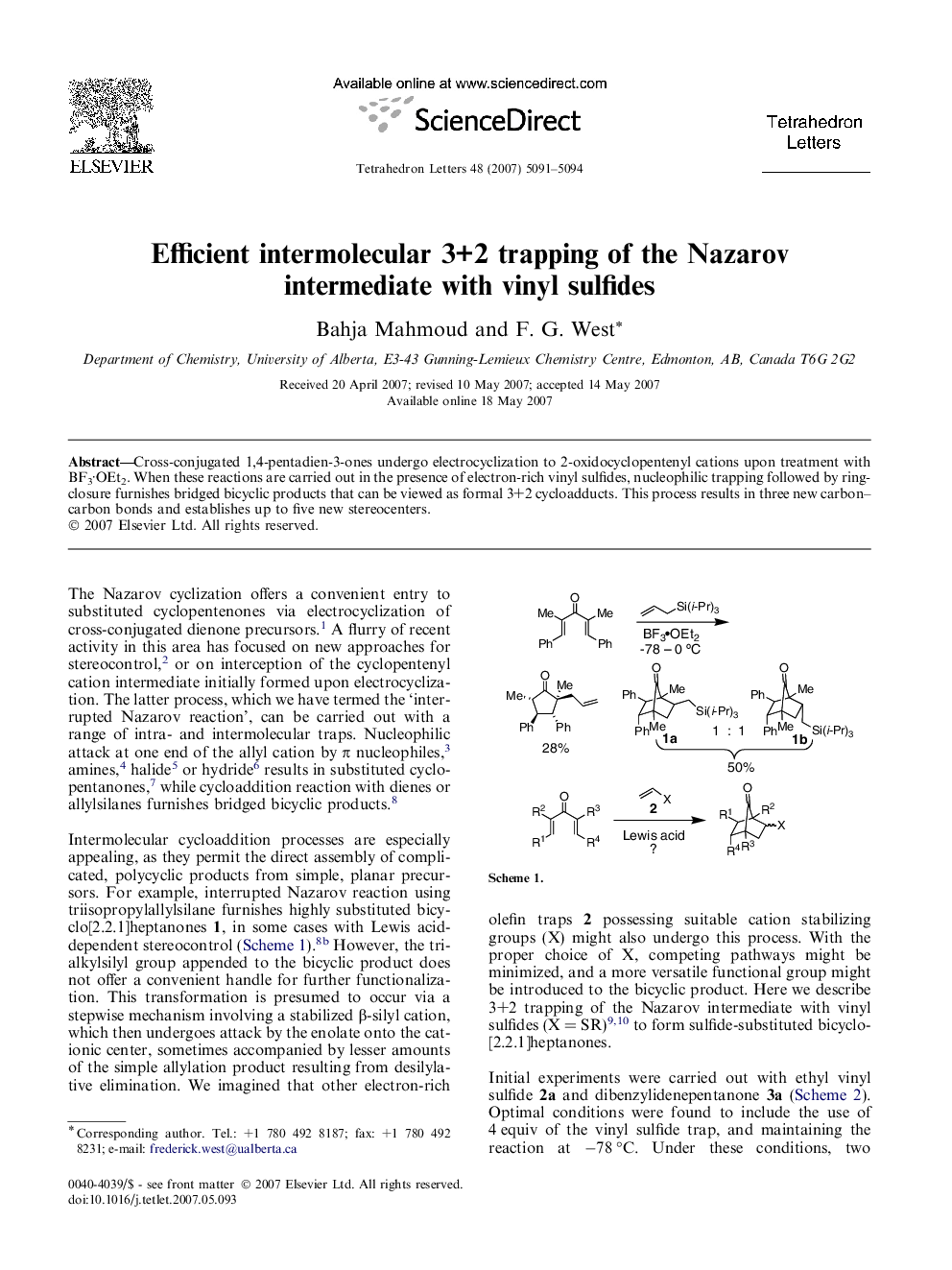
Simple cross-conjugated dienones undergo Nazarov cyclization and in situ trapping in the presence of vinyl sulfides, yielding functionalized bicyclo[2.2.1]heptanone products. The formal 3+2 cycloaddition is presumed to be a stepwise process, but furnishes the adducts with good diastereoselectivity.
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry
Authors
Bahja Mahmoud, F.G. West,