| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5285182 | Tetrahedron Letters | 2006 | 4 Pages |
Abstract
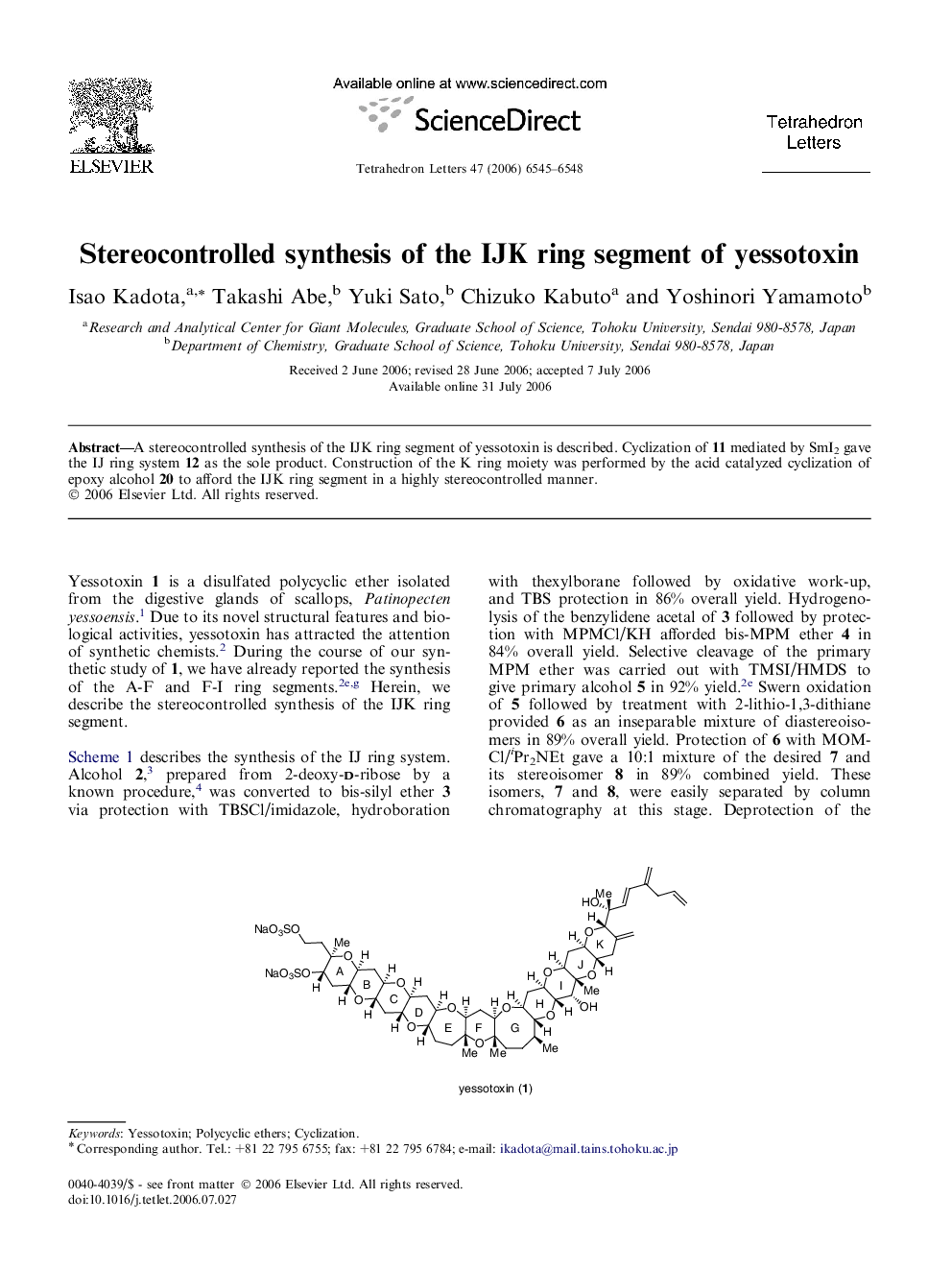
A stereocontrolled synthesis of the IJK ring segment of yessotoxin is described. Cyclization of 11 mediated by SmI2 gave the IJ ring system 12 as the sole product. Construction of the K ring moiety was performed by the acid catalyzed cyclization of epoxy alcohol 20 to afford the IJK ring segment in a highly stereocontrolled manner.
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry
Authors
Isao Kadota, Takashi Abe, Yuki Sato, Chizuko Kabuto, Yoshinori Yamamoto,