| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5288651 | Tetrahedron Letters | 2006 | 4 Pages |
Abstract
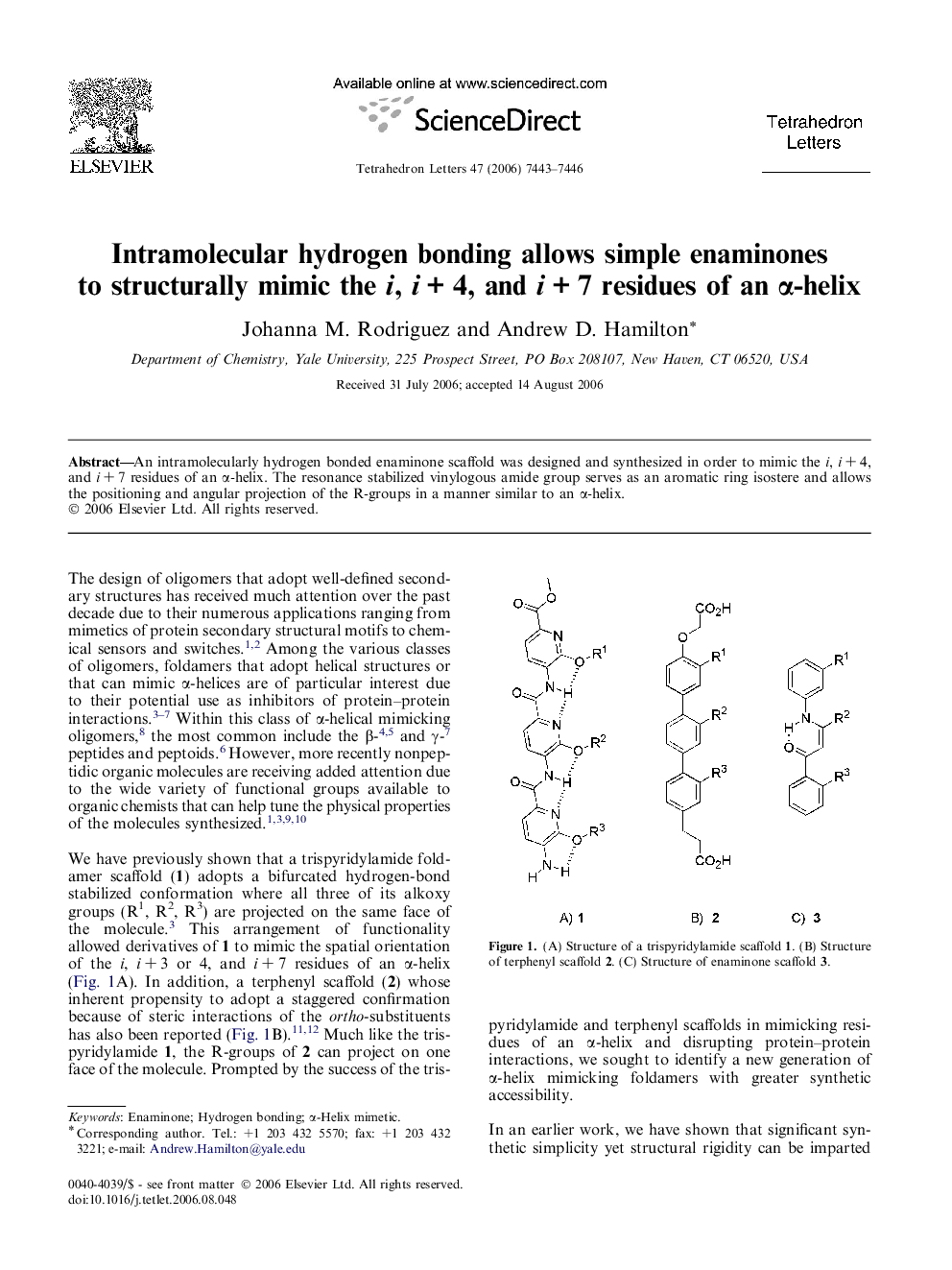
An intramolecularly hydrogen bonded enaminone scaffold was designed and synthesized in order to mimic the i, i + 4, and i + 7 residues of an α-helix. The conformationally rigid vinylogous amide group serves as an aromatic ring isostere and allows the positioning and angular projection of the R-groups in a manner similar to an α-helix.
Keywords
Related Topics
Physical Sciences and Engineering
Chemistry
Organic Chemistry
Authors
Johanna M. Rodriguez, Andrew D. Hamilton,