| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5370597 | Biophysical Chemistry | 2017 | 6 Pages |
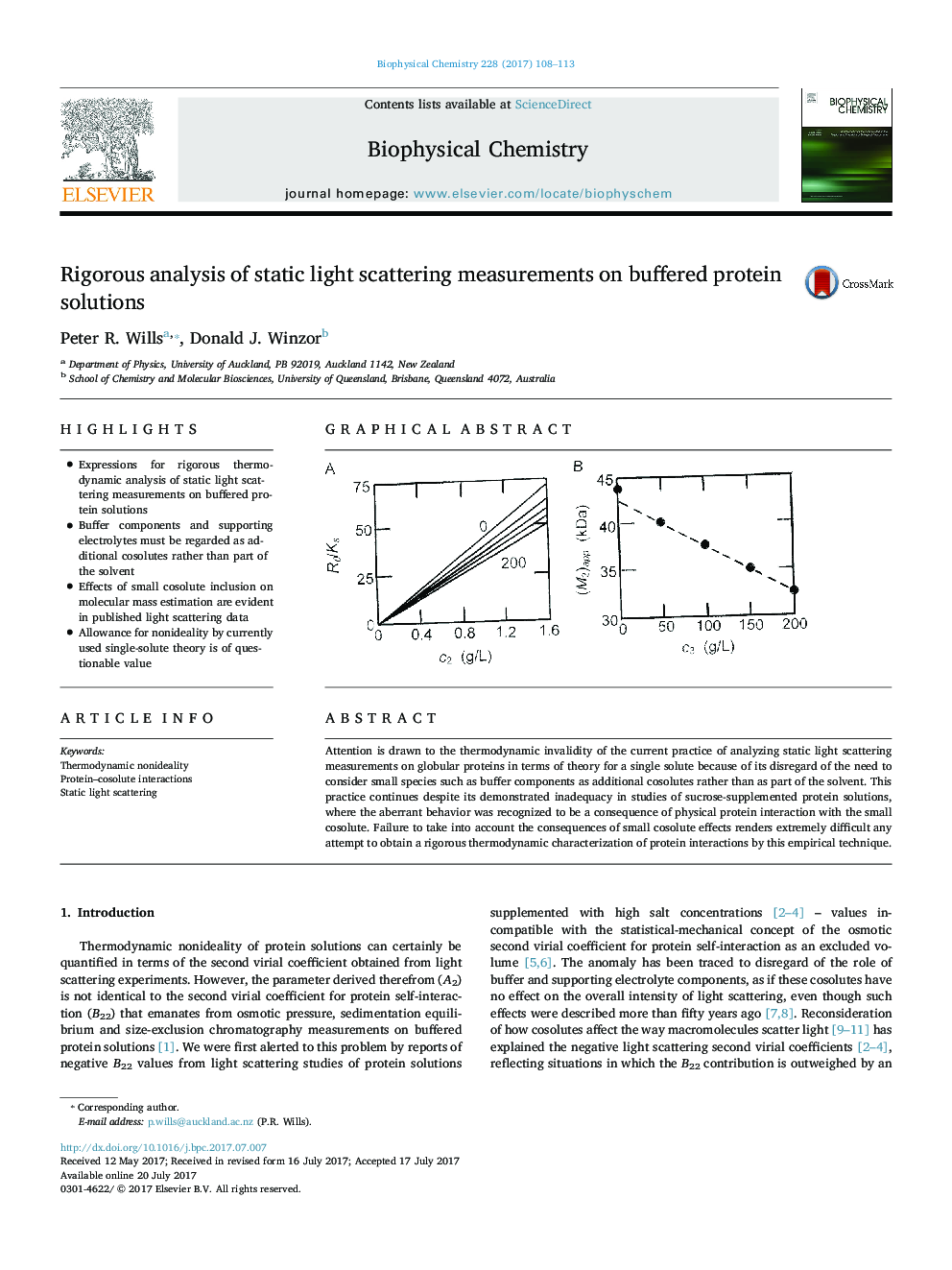
â¢Expressions for rigorous thermodynamic analysis of static light scattering measurements on buffered protein solutionsâ¢Buffer components and supporting electrolytes must be regarded as additional cosolutes rather than part of the solventâ¢Effects of small cosolute inclusion on molecular mass estimation are evident in published light scattering dataâ¢Allowance for nonideality by currently used single-solute theory is of questionable value
Attention is drawn to the thermodynamic invalidity of the current practice of analyzing static light scattering measurements on globular proteins in terms of theory for a single solute because of its disregard of the need to consider small species such as buffer components as additional cosolutes rather than as part of the solvent. This practice continues despite its demonstrated inadequacy in studies of sucrose-supplemented protein solutions, where the aberrant behavior was recognized to be a consequence of physical protein interaction with the small cosolute. Failure to take into account the consequences of small cosolute effects renders extremely difficult any attempt to obtain a rigorous thermodynamic characterization of protein interactions by this empirical technique.
Graphical abstractDownload high-res image (176KB)Download full-size image