| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5370602 | Biophysical Chemistry | 2017 | 8 Pages |
â¢Following the aggregation process of Aβ1-42 peptides by scattering techniques.â¢Punctual mutations in Aβ1-42 give rise to aggregates different in size and number.â¢Interaction of Aβ1-42 A2V and A2T peptides with membranes and cells.
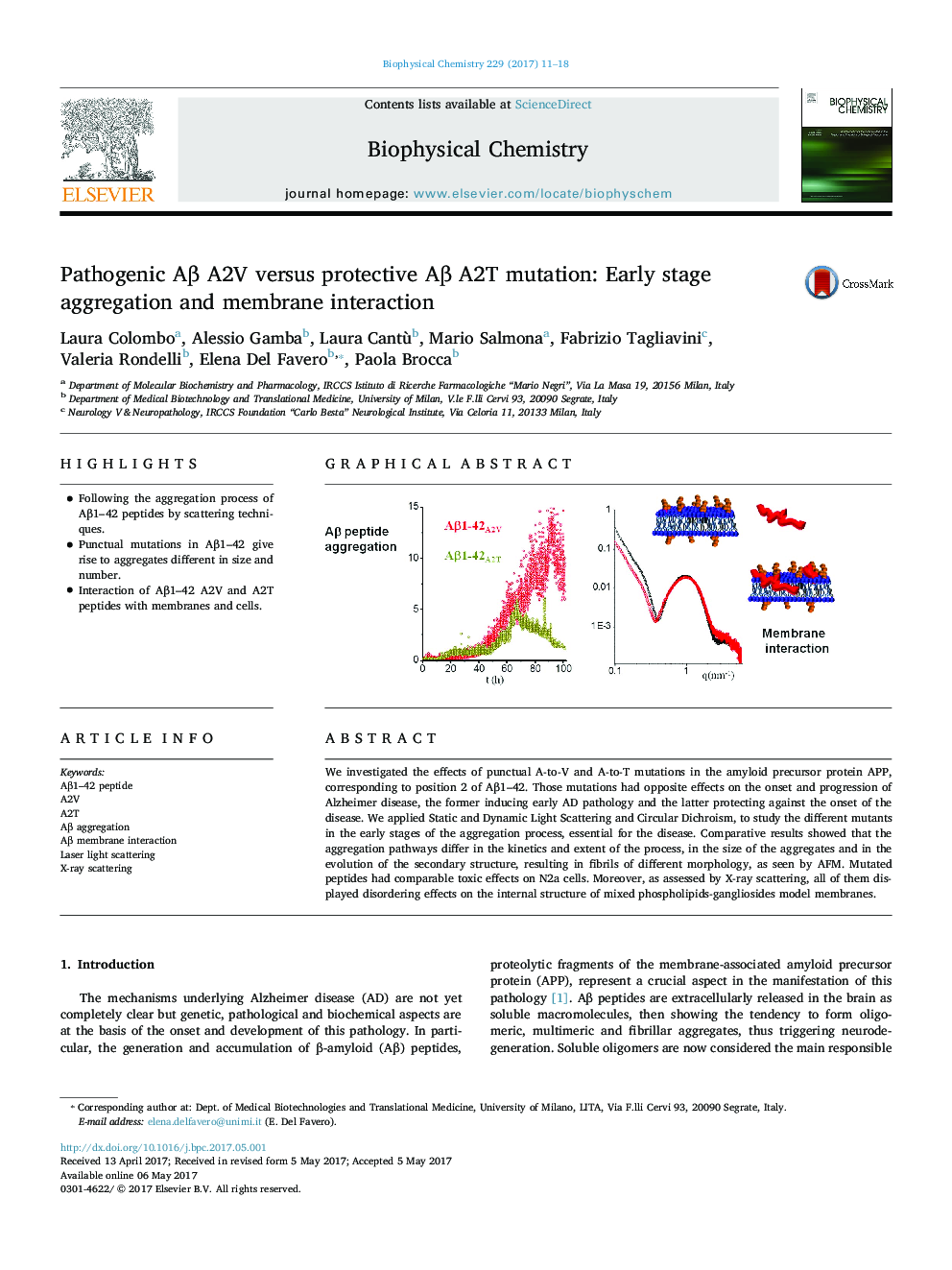
We investigated the effects of punctual A-to-V and A-to-T mutations in the amyloid precursor protein APP, corresponding to position 2 of Aβ1-42. Those mutations had opposite effects on the onset and progression of Alzheimer disease, the former inducing early AD pathology and the latter protecting against the onset of the disease. We applied Static and Dynamic Light Scattering and Circular Dichroism, to study the different mutants in the early stages of the aggregation process, essential for the disease. Comparative results showed that the aggregation pathways differ in the kinetics and extent of the process, in the size of the aggregates and in the evolution of the secondary structure, resulting in fibrils of different morphology, as seen by AFM. Mutated peptides had comparable toxic effects on N2a cells. Moreover, as assessed by X-ray scattering, all of them displayed disordering effects on the internal structure of mixed phospholipids-gangliosides model membranes.
Graphical abstractDownload high-res image (162KB)Download full-size image