| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5370701 | Biophysical Chemistry | 2016 | 4 Pages |
â¢We present a thermodynamic linkage analysis of pH-dependent i-motif stability.â¢It can be described by in terms of cytosine pKa's in the folded and unfolded states.â¢Differential number of protons bound to i-motif is calculated as a function of pH.â¢Cosolvent effect on the transition midpoint pH is rationalized.
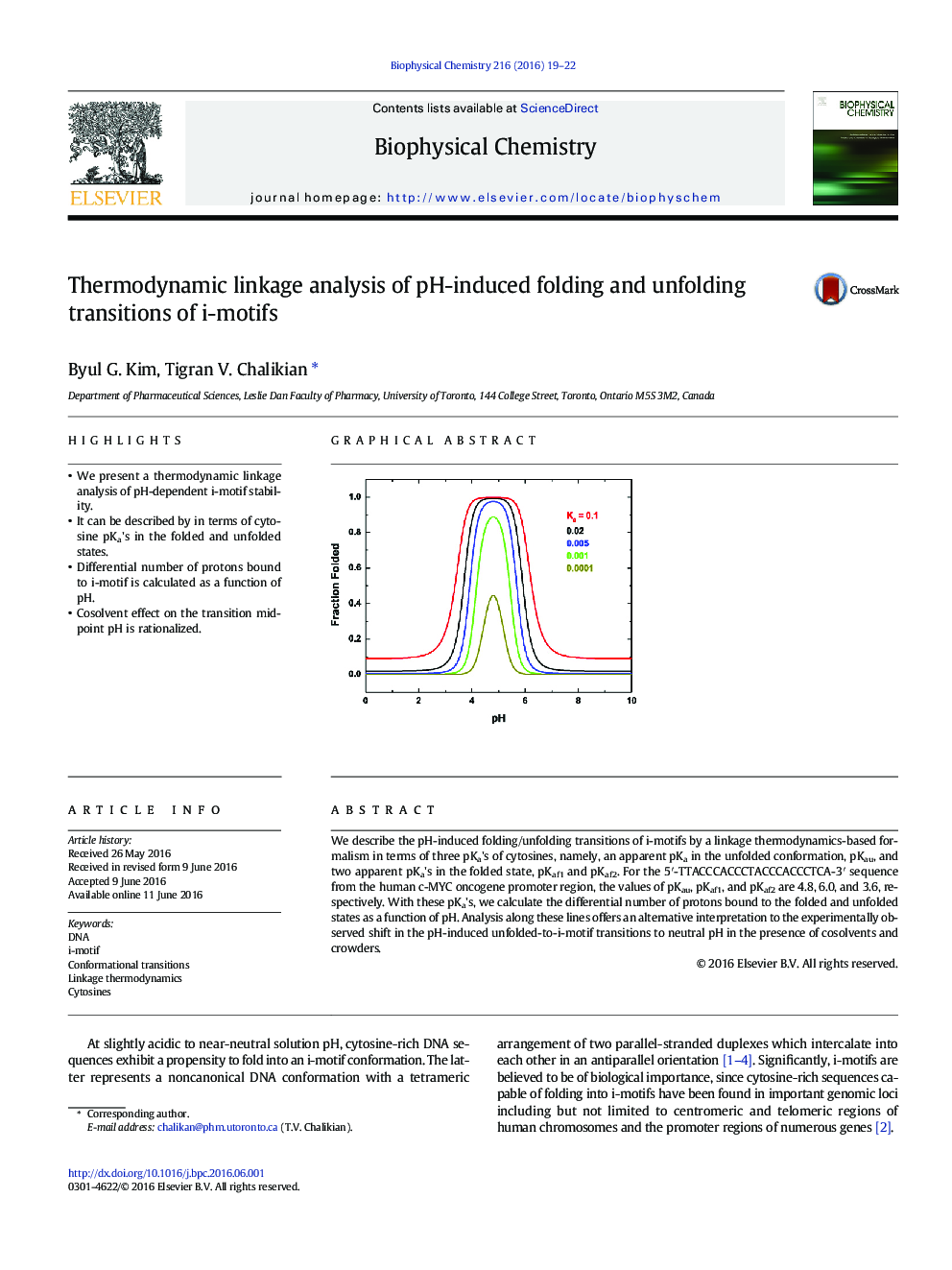
We describe the pH-induced folding/unfolding transitions of i-motifs by a linkage thermodynamics-based formalism in terms of three pKa's of cytosines, namely, an apparent pKa in the unfolded conformation, pKau, and two apparent pKa's in the folded state, pKaf1 and pKaf2. For the 5â²-TTACCCACCCTACCCACCCTCA-3â² sequence from the human c-MYC oncogene promoter region, the values of pKau, pKaf1, and pKaf2 are 4.8, 6.0, and 3.6, respectively. With these pKa's, we calculate the differential number of protons bound to the folded and unfolded states as a function of pH. Analysis along these lines offers an alternative interpretation to the experimentally observed shift in the pH-induced unfolded-to-i-motif transitions to neutral pH in the presence of cosolvents and crowders.
Graphical abstractDownload full-size image