| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5370984 | Biophysical Chemistry | 2014 | 11 Pages |
â¢Three mechanisms for competitive inhibition of protein aggregation are introduced.â¢Rate equations are derived to estimate aggregation inhibition constants.â¢Rate equations are used to distinguish inhibition mechanisms of insulin aggregation.â¢Longer insulin peptide inhibitors delay insulin aggregation onset.â¢Shorter insulin peptide inhibitors reduce total concentration of aggregated insulin.
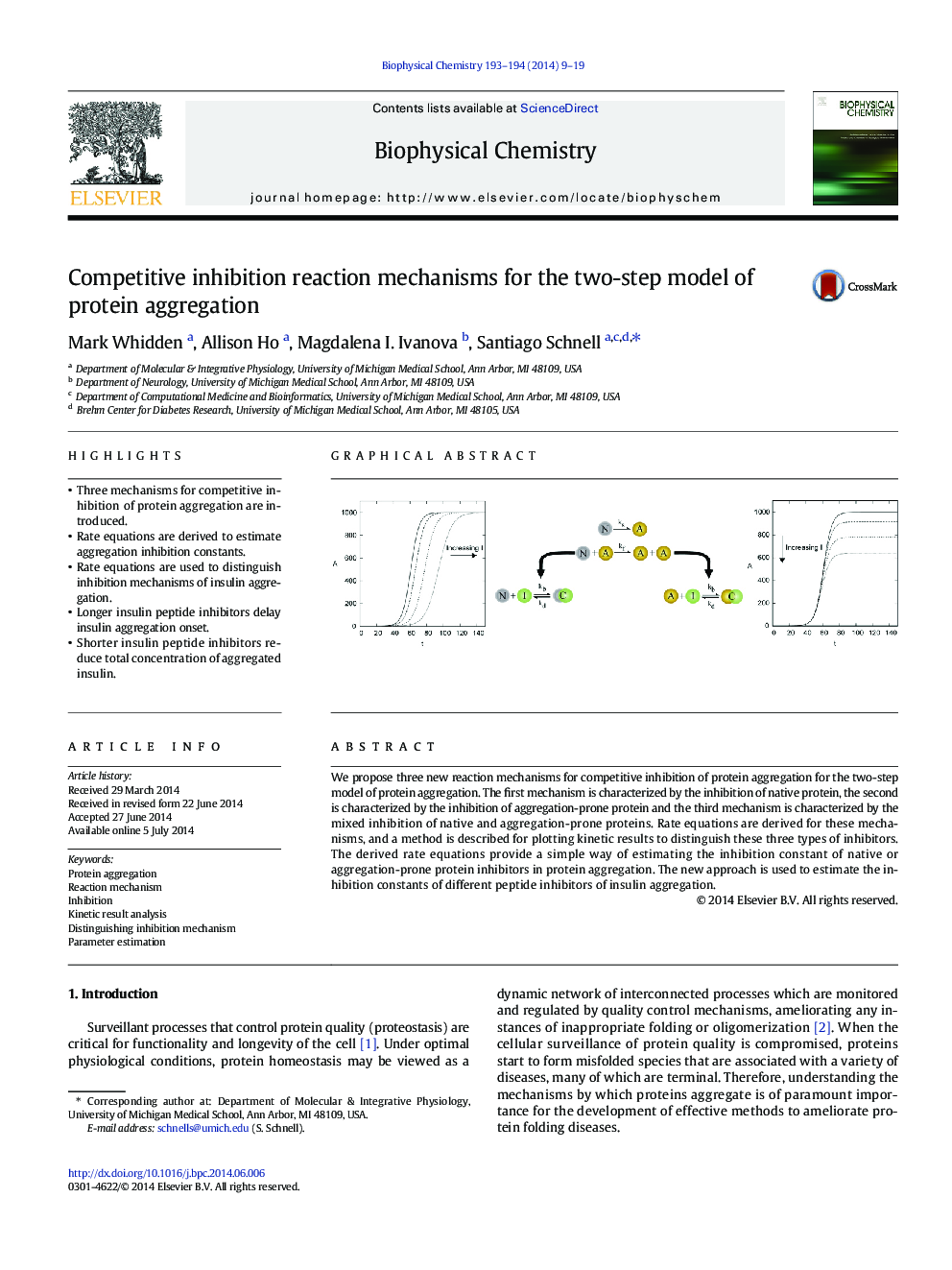
We propose three new reaction mechanisms for competitive inhibition of protein aggregation for the two-step model of protein aggregation. The first mechanism is characterized by the inhibition of native protein, the second is characterized by the inhibition of aggregation-prone protein and the third mechanism is characterized by the mixed inhibition of native and aggregation-prone proteins. Rate equations are derived for these mechanisms, and a method is described for plotting kinetic results to distinguish these three types of inhibitors. The derived rate equations provide a simple way of estimating the inhibition constant of native or aggregation-prone protein inhibitors in protein aggregation. The new approach is used to estimate the inhibition constants of different peptide inhibitors of insulin aggregation.
Graphical abstractDownload full-size image