| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5371123 | Biophysical Chemistry | 2013 | 12 Pages |
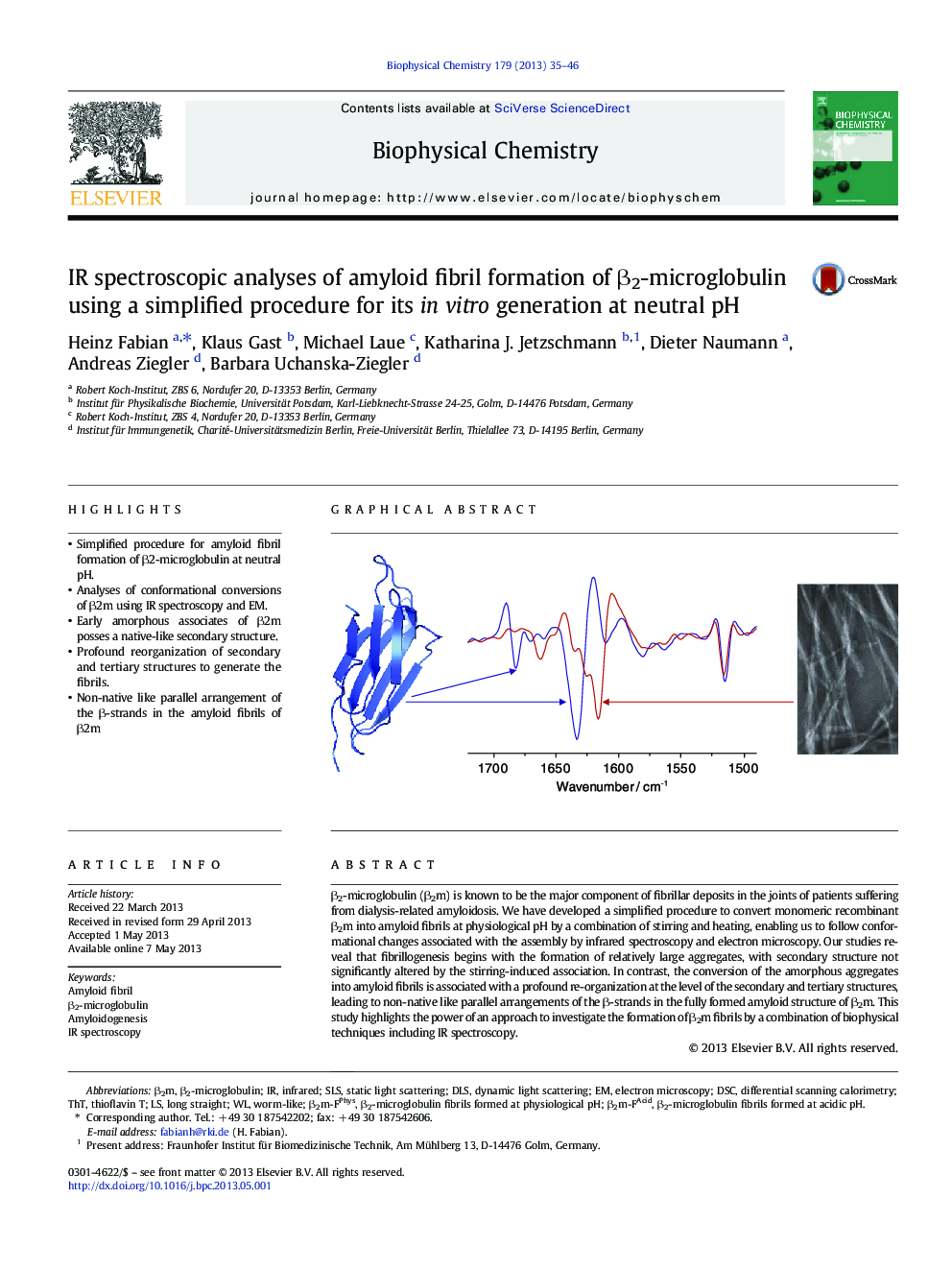
â¢Simplified procedure for amyloid fibril formation of β2-microglobulin at neutral pH.â¢Analyses of conformational conversions of β2m using IR spectroscopy and EM.â¢Early amorphous associates of β2m posses a native-like secondary structure.â¢Profound reorganization of secondary and tertiary structures to generate the fibrils.â¢Non-native like parallel arrangement of the β-strands in the amyloid fibrils of β2m
β2-microglobulin (β2m) is known to be the major component of fibrillar deposits in the joints of patients suffering from dialysis-related amyloidosis. We have developed a simplified procedure to convert monomeric recombinant β2m into amyloid fibrils at physiological pH by a combination of stirring and heating, enabling us to follow conformational changes associated with the assembly by infrared spectroscopy and electron microscopy. Our studies reveal that fibrillogenesis begins with the formation of relatively large aggregates, with secondary structure not significantly altered by the stirring-induced association. In contrast, the conversion of the amorphous aggregates into amyloid fibrils is associated with a profound re-organization at the level of the secondary and tertiary structures, leading to non-native like parallel arrangements of the β-strands in the fully formed amyloid structure of β2m. This study highlights the power of an approach to investigate the formation of β2m fibrils by a combination of biophysical techniques including IR spectroscopy.
Graphical abstractDownload full-size image