| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5371141 | Biophysical Chemistry | 2013 | 10 Pages |
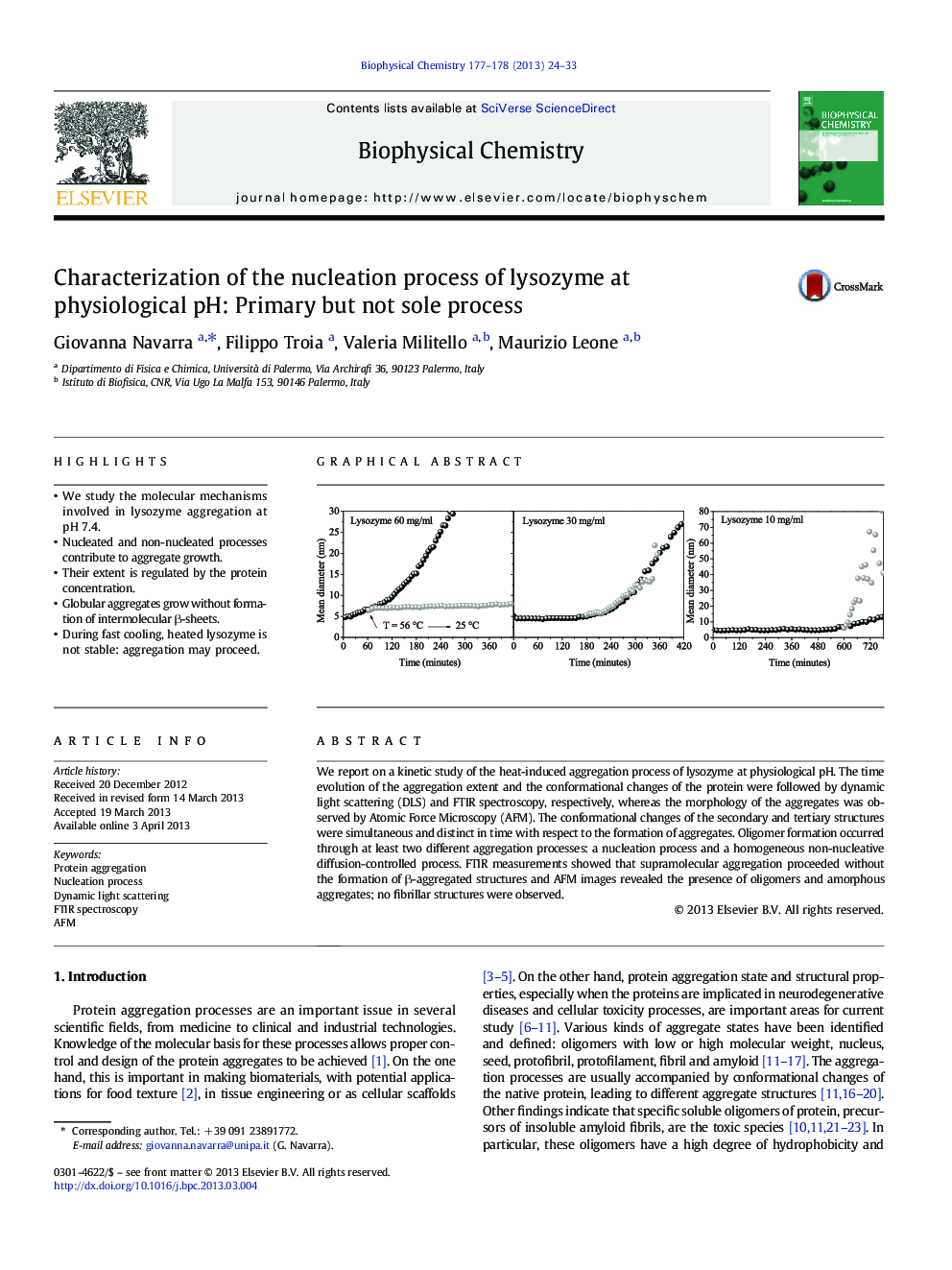
â¢We study the molecular mechanisms involved in lysozyme aggregation at pH 7.4.â¢Nucleated and non-nucleated processes contribute to aggregate growth.â¢Their extent is regulated by the protein concentration.â¢Globular aggregates grow without formation of intermolecular β-sheets.â¢During fast cooling, heated lysozyme is not stable: aggregation may proceed.
We report on a kinetic study of the heat-induced aggregation process of lysozyme at physiological pH. The time evolution of the aggregation extent and the conformational changes of the protein were followed by dynamic light scattering (DLS) and FTIR spectroscopy, respectively, whereas the morphology of the aggregates was observed by Atomic Force Microscopy (AFM). The conformational changes of the secondary and tertiary structures were simultaneous and distinct in time with respect to the formation of aggregates. Oligomer formation occurred through at least two different aggregation processes: a nucleation process and a homogeneous non-nucleative diffusion-controlled process. FTIR measurements showed that supramolecular aggregation proceeded without the formation of β-aggregated structures and AFM images revealed the presence of oligomers and amorphous aggregates; no fibrillar structures were observed.
Graphical abstractDownload full-size image