| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5371153 | Biophysical Chemistry | 2013 | 7 Pages |
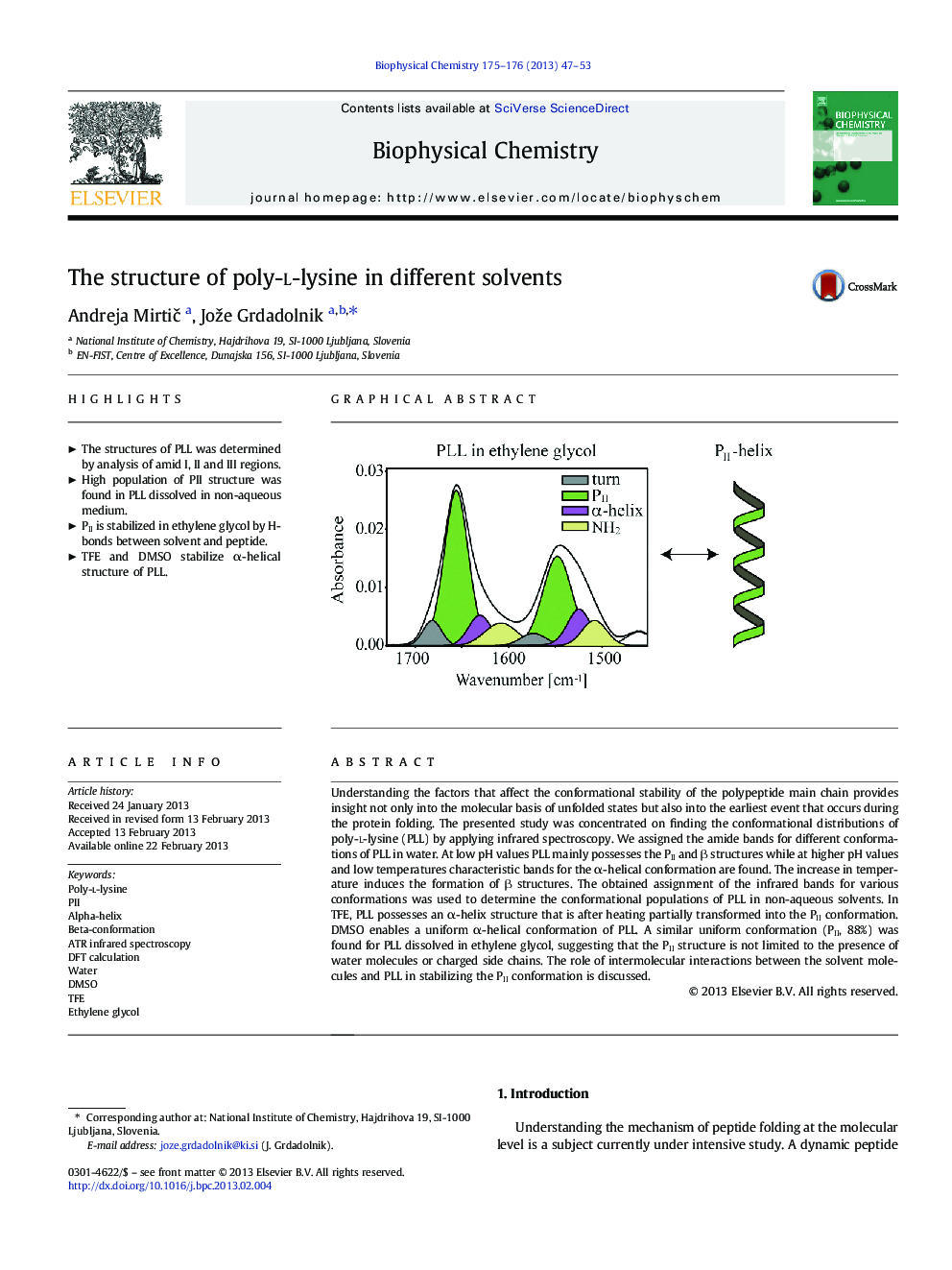
Understanding the factors that affect the conformational stability of the polypeptide main chain provides insight not only into the molecular basis of unfolded states but also into the earliest event that occurs during the protein folding. The presented study was concentrated on finding the conformational distributions of poly-l-lysine (PLL) by applying infrared spectroscopy. We assigned the amide bands for different conformations of PLL in water. At low pH values PLL mainly possesses the PII and β structures while at higher pH values and low temperatures characteristic bands for the α-helical conformation are found. The increase in temperature induces the formation of β structures. The obtained assignment of the infrared bands for various conformations was used to determine the conformational populations of PLL in non-aqueous solvents. In TFE, PLL possesses an α-helix structure that is after heating partially transformed into the PII conformation. DMSO enables a uniform α-helical conformation of PLL. A similar uniform conformation (PII, 88%) was found for PLL dissolved in ethylene glycol, suggesting that the PII structure is not limited to the presence of water molecules or charged side chains. The role of intermolecular interactions between the solvent molecules and PLL in stabilizing the PII conformation is discussed.
Graphical abstractDownload full-size imageHighlights⺠The structures of PLL was determined by analysis of amid I, II and III regions. ⺠High population of PII structure was found in PLL dissolved in non-aqueous medium. ⺠PII is stabilized in ethylene glycol by H-bonds between solvent and peptide. ⺠TFE and DMSO stabilize α-helical structure of PLL.