| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5372704 | Chemical Physics | 2017 | 11 Pages |
â¢Series of complex perovskites Pb2MReO6 (M = Cr, Mn and Fe) have been investigated.â¢The occupation-numbers in M (3dn)-Re (5dm) influenced the physical properties.â¢Pb2MReO6 stabilized in tetragonal crystal (space group I4/m); (a0a0câ) tilt-system.â¢LSDA + U calculations produced ferrimagnetic half-metallic in all compounds.
Three compounds of lead-based complex perovskites Pb2MReO6 (M = Cr, Mn and Fe) have been investigated in detail based on density functional theory (DFT) using local spin density approximation (LSDA) and (LSDA + U) methods. By introducing a series of 3d-ions in M-site, the number of valence electrons that occupied the 3d-orbitals can be increased from Cr3+(3d3) to Mn2+(3d5) and Fe3+(3d5), and this beside the effect of energy U are the main factors that influenced the physical properties of Pb2MReO6. Magnetic and electronic calculations showed that all Pb2MReO6 compounds have ferrimagnetic half-metallic (FI-HM) properties. FI-HM are attributed to the M (3d)-Re (5d) hybridization through the strong 180° super-exchange (SE) interaction via the long-range pathway M (3d)â-O (2p)-Re (5d)â, in conformity with both Pauli Exclusion principles and Goodenough-Kanamori rules. This result is interpreted within a scenario where the Re (5d) states play a crucial role in the FI-HM ground state.
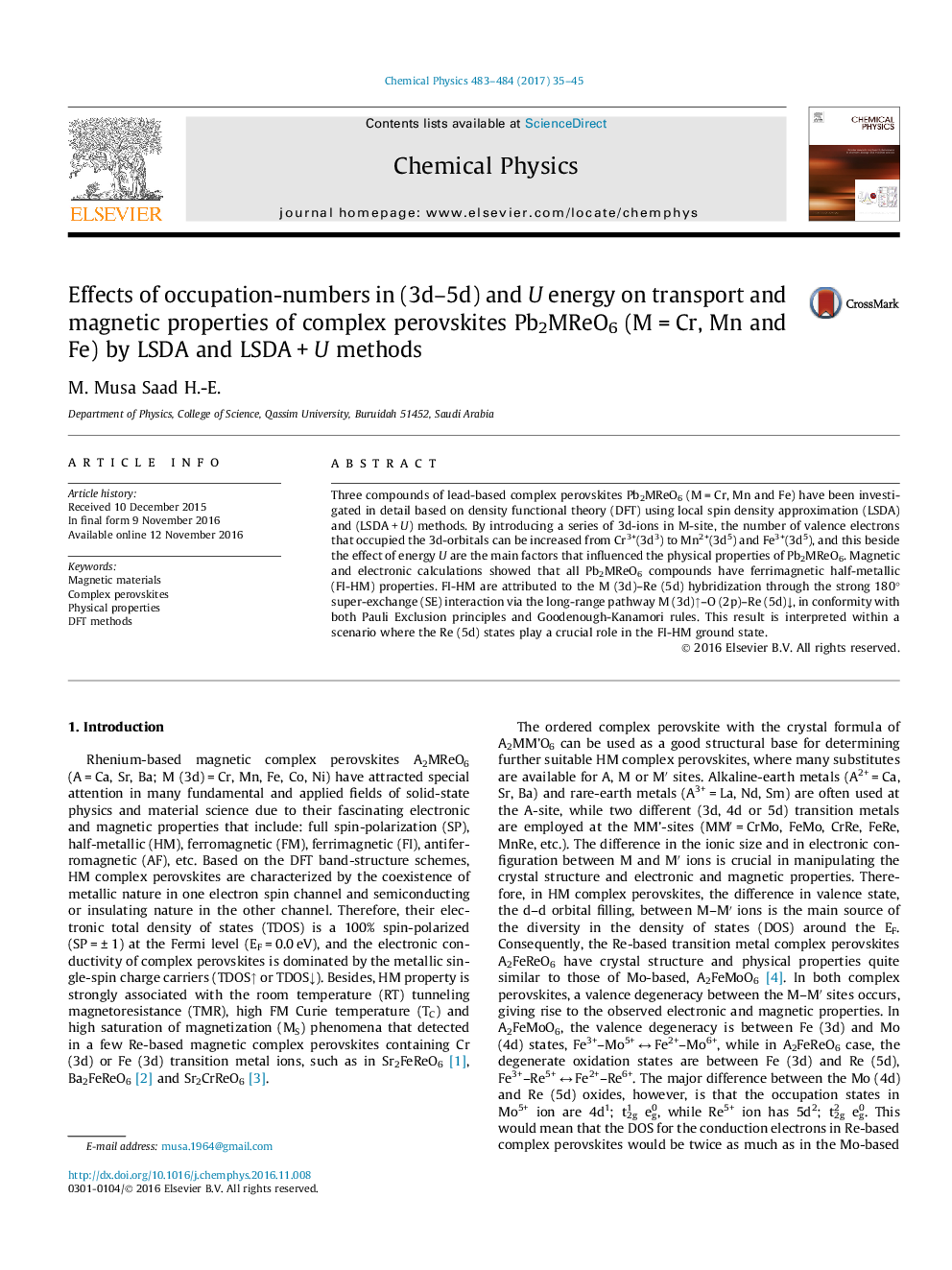
Graphical abstractSchematic illustration of the ferrimagnetic ordering between M (3d) and Re (5d) ions, the spin on Cr3+ (SÂ =Â 3/2) - Re5+ (SÂ =Â 1), Mn2+ (SÂ =Â 5/2) - Re6+ (SÂ =Â 1/2) and Fe3+ (SÂ =Â 5/2) - Re5+ (SÂ =Â 1) interacted antiferromagnetically by a strong super-exchange interaction through the O2â ligands, Mâ-O-Reâ, resulting in spontaneous magnetization. Small black bars inside the spheres represent the levels of the 3d and 5d orbitals on M (3d) and Re (5d) ions. Up and down arrows represent the spin-direction on M (3d) and Re (5d) ions.Download high-res image (69KB)Download full-size image