| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5621587 | Seminars in Thoracic and Cardiovascular Surgery | 2016 | 8 Pages |
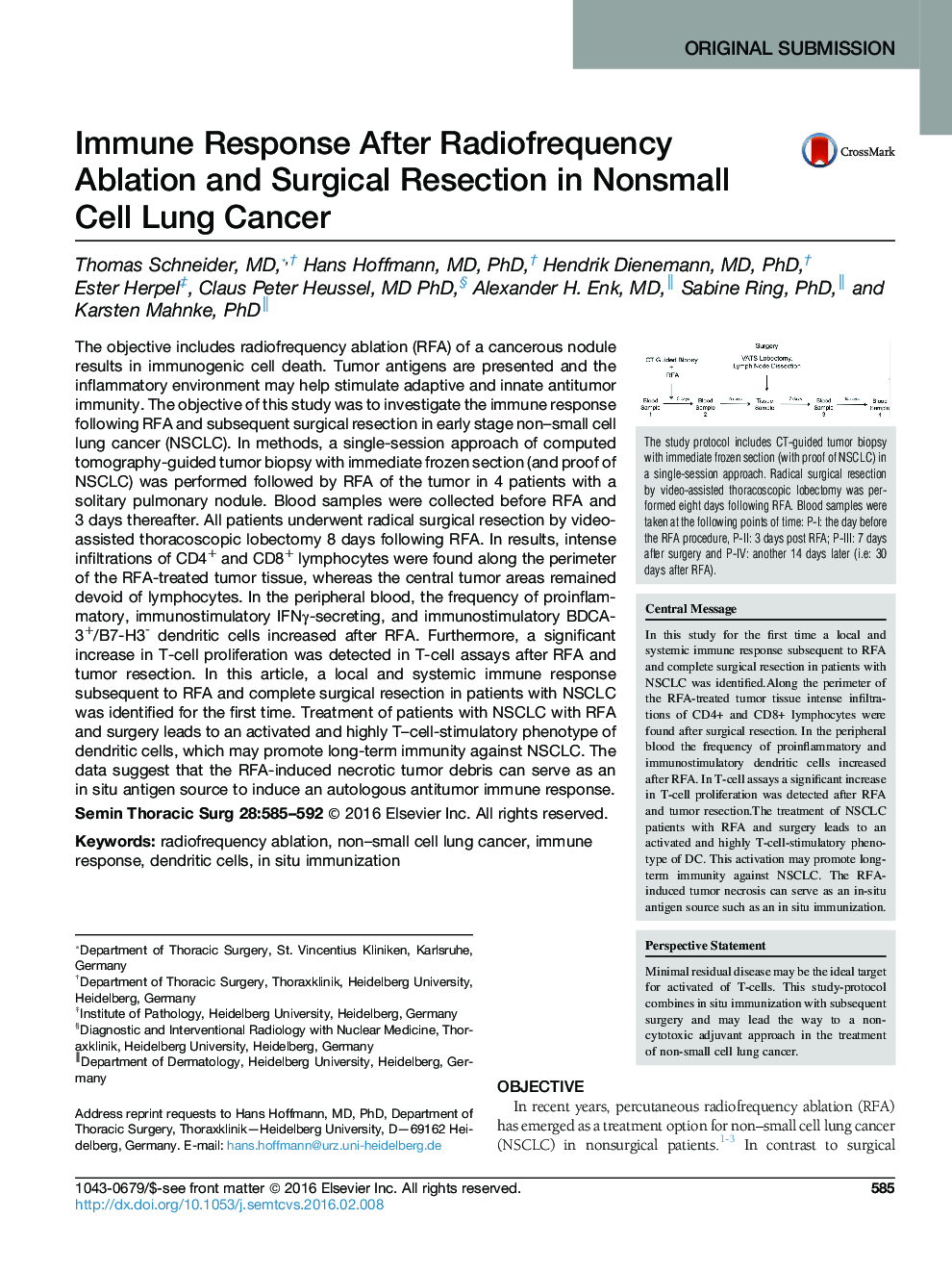
The objective includes radiofrequency ablation (RFA) of a cancerous nodule results in immunogenic cell death. Tumor antigens are presented and the inflammatory environment may help stimulate adaptive and innate antitumor immunity. The objective of this study was to investigate the immune response following RFA and subsequent surgical resection in early stage non-small cell lung cancer (NSCLC). In methods, a single-session approach of computed tomography-guided tumor biopsy with immediate frozen section (and proof of NSCLC) was performed followed by RFA of the tumor in 4 patients with a solitary pulmonary nodule. Blood samples were collected before RFA and 3 days thereafter. All patients underwent radical surgical resection by video-assisted thoracoscopic lobectomy 8 days following RFA. In results, intense infiltrations of CD4+ and CD8+ lymphocytes were found along the perimeter of the RFA-treated tumor tissue, whereas the central tumor areas remained devoid of lymphocytes. In the peripheral blood, the frequency of proinflammatory, immunostimulatory IFNγ-secreting, and immunostimulatory BDCA-3+/B7-H3- dendritic cells increased after RFA. Furthermore, a significant increase in T-cell proliferation was detected in T-cell assays after RFA and tumor resection. In this article, a local and systemic immune response subsequent to RFA and complete surgical resection in patients with NSCLC was identified for the first time. Treatment of patients with NSCLC with RFA and surgery leads to an activated and highly T-cell-stimulatory phenotype of dendritic cells, which may promote long-term immunity against NSCLC. The data suggest that the RFA-induced necrotic tumor debris can serve as an in situ antigen source to induce an autologous antitumor immune response.