| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5746639 | Chemosphere | 2017 | 6 Pages |
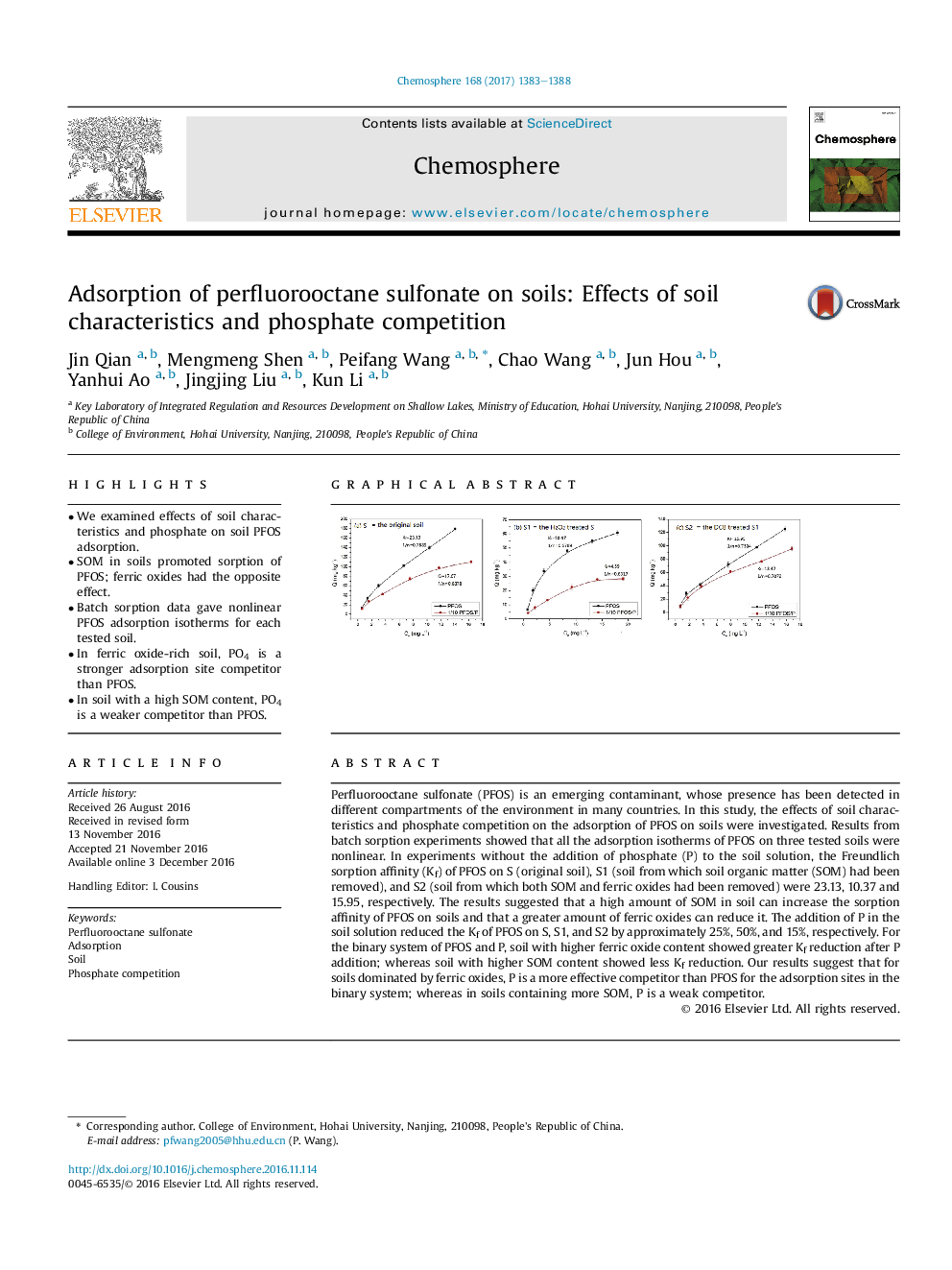
â¢We examined effects of soil characteristics and phosphate on soil PFOS adsorption.â¢SOM in soils promoted sorption of PFOS; ferric oxides had the opposite effect.â¢Batch sorption data gave nonlinear PFOS adsorption isotherms for each tested soil.â¢In ferric oxide-rich soil, PO4 is a stronger adsorption site competitor than PFOS.â¢In soil with a high SOM content, PO4 is a weaker competitor than PFOS.
Perfluorooctane sulfonate (PFOS) is an emerging contaminant, whose presence has been detected in different compartments of the environment in many countries. In this study, the effects of soil characteristics and phosphate competition on the adsorption of PFOS on soils were investigated. Results from batch sorption experiments showed that all the adsorption isotherms of PFOS on three tested soils were nonlinear. In experiments without the addition of phosphate (P) to the soil solution, the Freundlich sorption affinity (Kf) of PFOS on S (original soil), S1 (soil from which soil organic matter (SOM) had been removed), and S2 (soil from which both SOM and ferric oxides had been removed) were 23.13, 10.37 and 15.95, respectively. The results suggested that a high amount of SOM in soil can increase the sorption affinity of PFOS on soils and that a greater amount of ferric oxides can reduce it. The addition of P in the soil solution reduced the Kf of PFOS on S, S1, and S2 by approximately 25%, 50%, and 15%, respectively. For the binary system of PFOS and P, soil with higher ferric oxide content showed greater Kf reduction after P addition; whereas soil with higher SOM content showed less Kf reduction. Our results suggest that for soils dominated by ferric oxides, P is a more effective competitor than PFOS for the adsorption sites in the binary system; whereas in soils containing more SOM, P is a weak competitor.
Graphical abstractDownload high-res image (164KB)Download full-size image