| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5746670 | Chemosphere | 2017 | 7 Pages |
â¢Interaction of surfactants with different head groups with DNA are studied.â¢Binding modes are obtained by spectroscopy, viscosity and gel electrophoresis.â¢Interaction strength of DNA and surfactants with different head groups are compared.â¢Effects of concentration, ratio, and ionic strength on interaction are assessed.
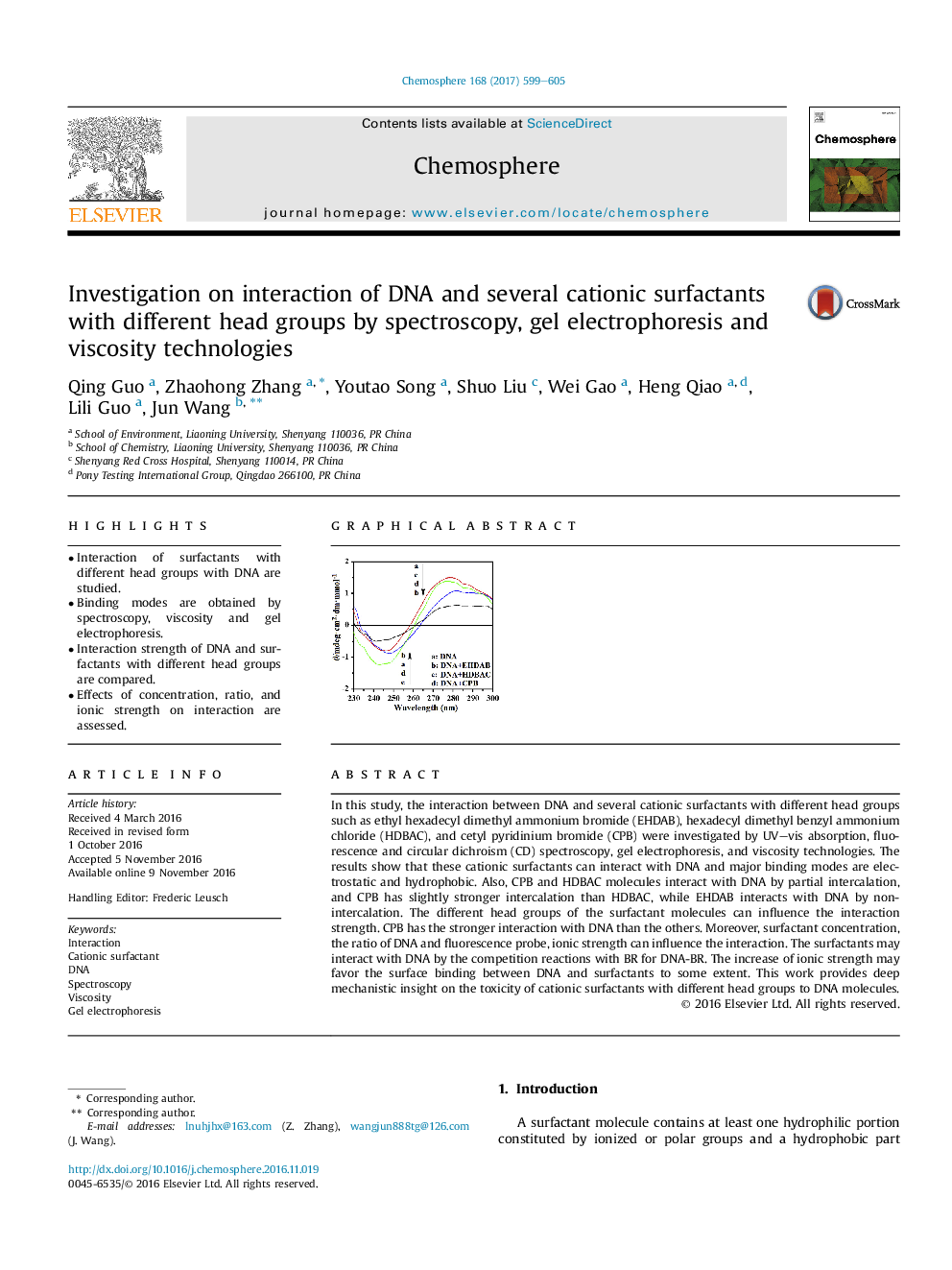
In this study, the interaction between DNA and several cationic surfactants with different head groups such as ethyl hexadecyl dimethyl ammonium bromide (EHDAB), hexadecyl dimethyl benzyl ammonium chloride (HDBAC), and cetyl pyridinium bromide (CPB) were investigated by UV-vis absorption, fluorescence and circular dichroism (CD) spectroscopy, gel electrophoresis, and viscosity technologies. The results show that these cationic surfactants can interact with DNA and major binding modes are electrostatic and hydrophobic. Also, CPB and HDBAC molecules interact with DNA by partial intercalation, and CPB has slightly stronger intercalation than HDBAC, while EHDAB interacts with DNA by non-intercalation. The different head groups of the surfactant molecules can influence the interaction strength. CPB has the stronger interaction with DNA than the others. Moreover, surfactant concentration, the ratio of DNA and fluorescence probe, ionic strength can influence the interaction. The surfactants may interact with DNA by the competition reactions with BR for DNA-BR. The increase of ionic strength may favor the surface binding between DNA and surfactants to some extent. This work provides deep mechanistic insight on the toxicity of cationic surfactants with different head groups to DNA molecules.
Graphical abstractDownload high-res image (229KB)Download full-size image