| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5746708 | Chemosphere | 2017 | 9 Pages |
â¢Photodegradation tendencies of polybrominated diphenyl ethers (PBDEs) were analysed.â¢The effects of each substituent position on photodegradation of PBDEs were studied.â¢Full factorial experimental design was used to study substituent characteristics.â¢The effect of solvents on the photodegradation of PBDEs was analysed.
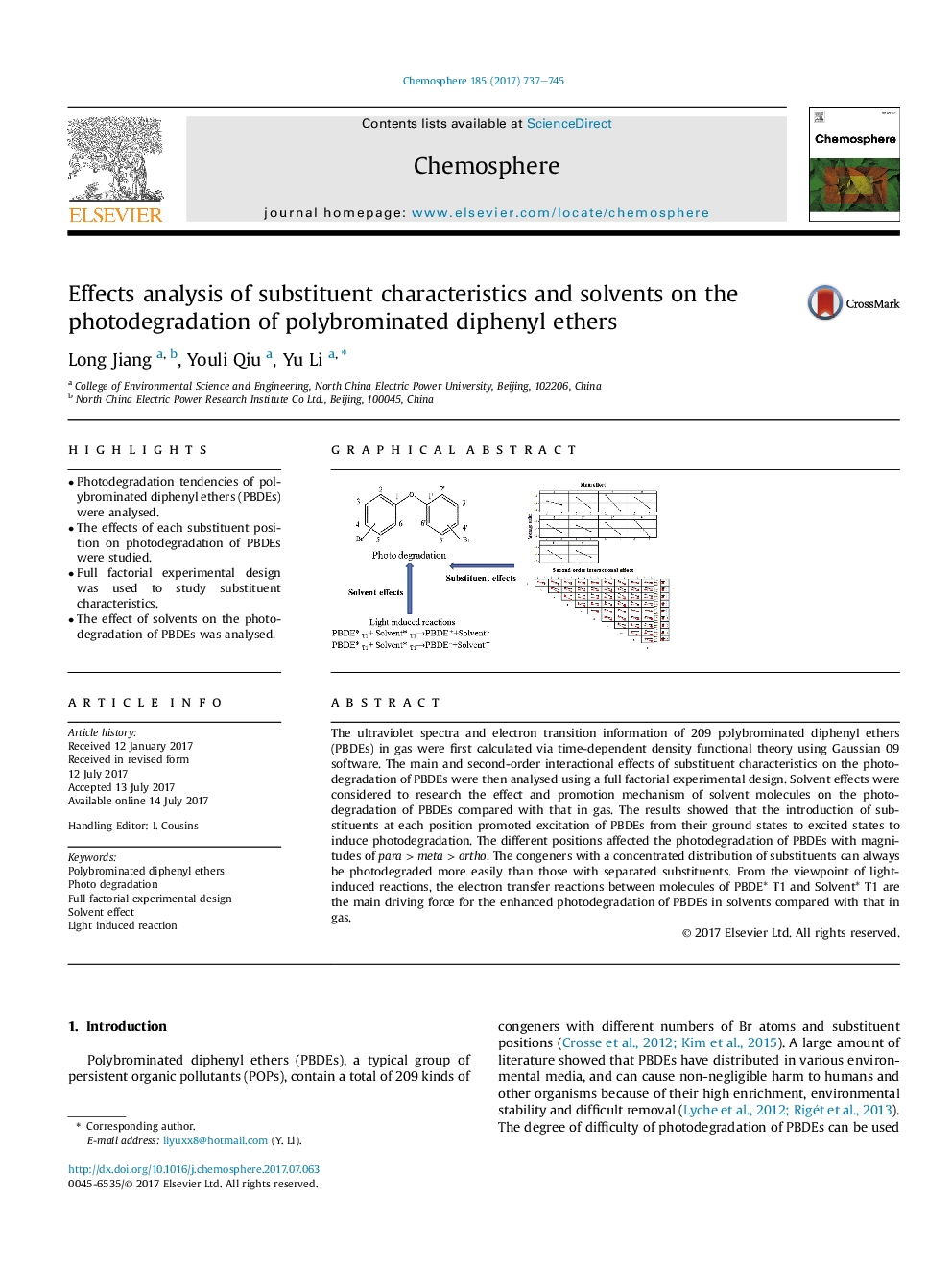
The ultraviolet spectra and electron transition information of 209 polybrominated diphenyl ethers (PBDEs) in gas were first calculated via time-dependent density functional theory using Gaussian 09 software. The main and second-order interactional effects of substituent characteristics on the photodegradation of PBDEs were then analysed using a full factorial experimental design. Solvent effects were considered to research the effect and promotion mechanism of solvent molecules on the photodegradation of PBDEs compared with that in gas. The results showed that the introduction of substituents at each position promoted excitation of PBDEs from their ground states to excited states to induce photodegradation. The different positions affected the photodegradation of PBDEs with magnitudes of para > meta > ortho. The congeners with a concentrated distribution of substituents can always be photodegraded more easily than those with separated substituents. From the viewpoint of light-induced reactions, the electron transfer reactions between molecules of PBDE* T1 and Solvent* T1 are the main driving force for the enhanced photodegradation of PBDEs in solvents compared with that in gas.
Graphical abstractDownload high-res image (239KB)Download full-size image