| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5747048 | Chemosphere | 2017 | 8 Pages |
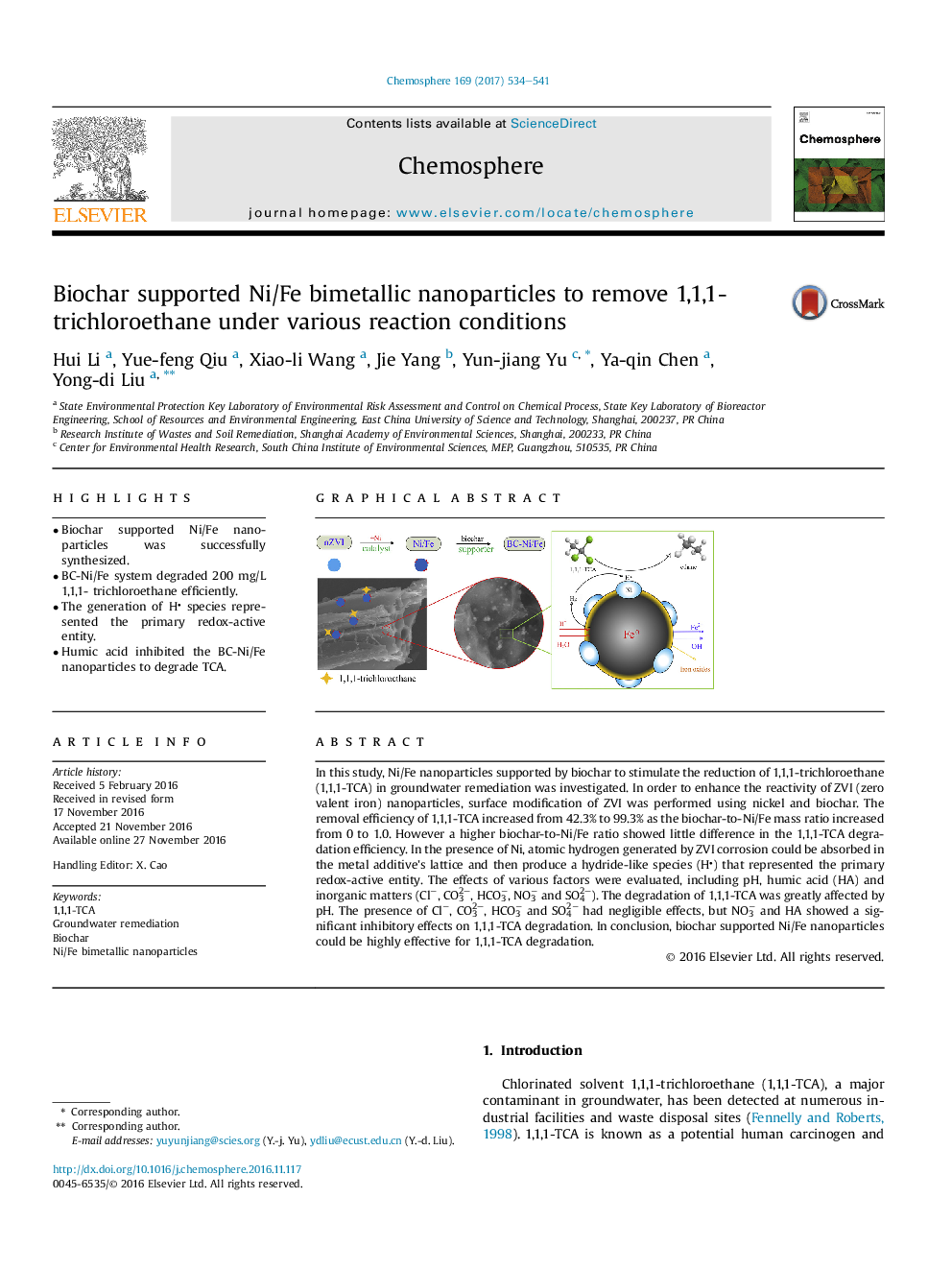
â¢Biochar supported Ni/Fe nanoparticles was successfully synthesized.â¢BC-Ni/Fe system degraded 200 mg/L 1,1,1- trichloroethane efficiently.â¢The generation of H species represented the primary redox-active entity.â¢Humic acid inhibited the BC-Ni/Fe nanoparticles to degrade TCA.
In this study, Ni/Fe nanoparticles supported by biochar to stimulate the reduction of 1,1,1-trichloroethane (1,1,1-TCA) in groundwater remediation was investigated. In order to enhance the reactivity of ZVI (zero valent iron) nanoparticles, surface modification of ZVI was performed using nickel and biochar. The removal efficiency of 1,1,1-TCA increased from 42.3% to 99.3% as the biochar-to-Ni/Fe mass ratio increased from 0 to 1.0. However a higher biochar-to-Ni/Fe ratio showed little difference in the 1,1,1-TCA degradation efficiency. In the presence of Ni, atomic hydrogen generated by ZVI corrosion could be absorbed in the metal additive's lattice and then produce a hydride-like species (H) that represented the primary redox-active entity. The effects of various factors were evaluated, including pH, humic acid (HA) and inorganic matters (Clâ, CO32-, HCO3â, NO3â and SO42-). The degradation of 1,1,1-TCA was greatly affected by pH. The presence of Clâ, CO32-, HCO3â and SO42â had negligible effects, but NO3â and HA showed a significant inhibitory effects on 1,1,1-TCA degradation. In conclusion, biochar supported Ni/Fe nanoparticles could be highly effective for 1,1,1-TCA degradation.
Graphical abstractDownload high-res image (323KB)Download full-size image