| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 5749592 | Environmental Technology & Innovation | 2017 | 13 Pages |
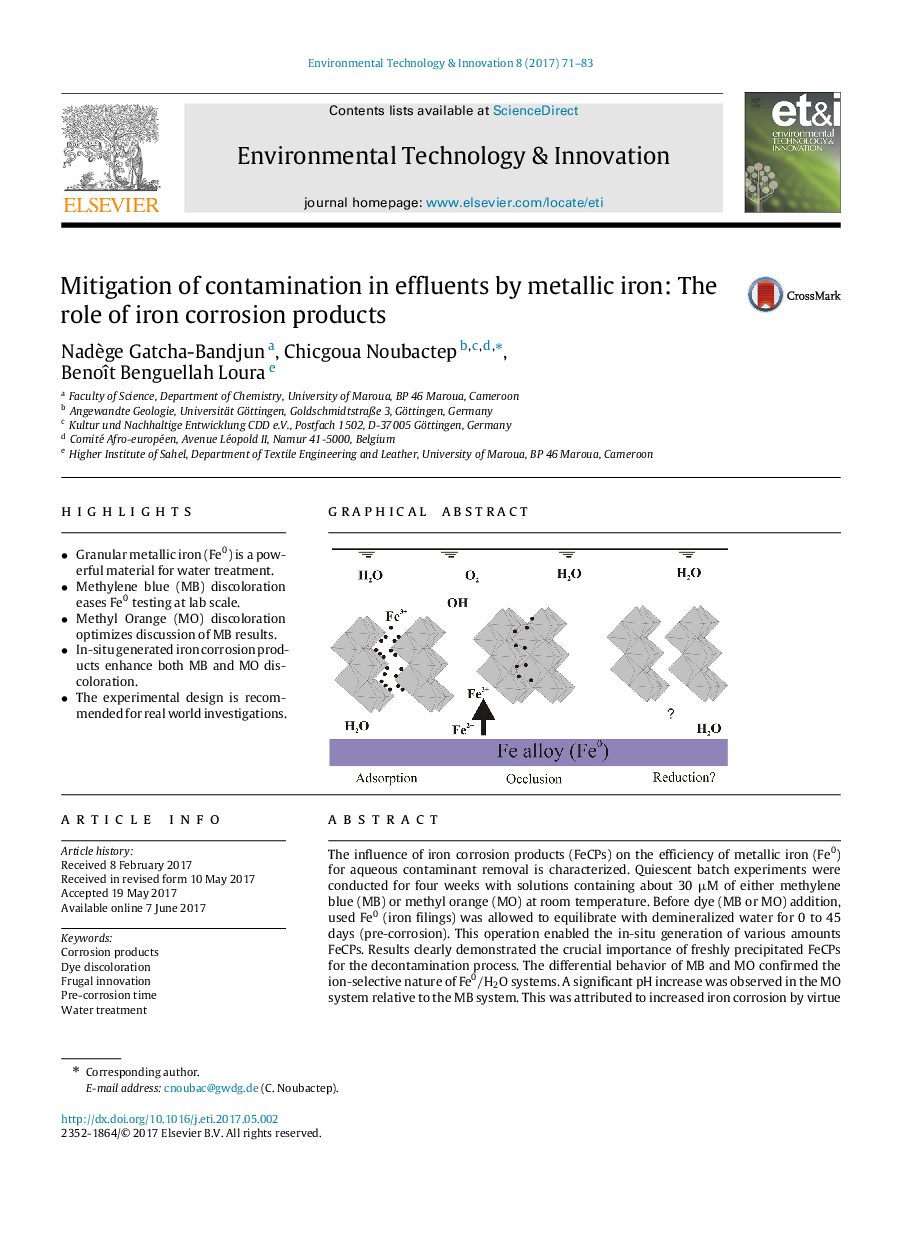
â¢Granular metallic iron (Fe0) is a powerful material for water treatment.â¢Methylene blue (MB) discoloration eases Fe0 testing at lab scale.â¢Methyl Orange (MO) discoloration optimizes discussion of MB results.â¢In-situ generated iron corrosion products enhance both MB and MO discoloration.â¢The experimental design is recommended for real world investigations.
The influence of iron corrosion products (FeCPs) on the efficiency of metallic iron (Fe0) for aqueous contaminant removal is characterized. Quiescent batch experiments were conducted for four weeks with solutions containing about 30μM of either methylene blue (MB) or methyl orange (MO) at room temperature. Before dye (MB or MO) addition, used Fe0 (iron filings) was allowed to equilibrate with demineralized water for 0 to 45 days (pre-corrosion). This operation enabled the in-situ generation of various amounts FeCPs. Results clearly demonstrated the crucial importance of freshly precipitated FeCPs for the decontamination process. The differential behavior of MB and MO confirmed the ion-selective nature of Fe0âH2O systems. A significant pH increase was observed in the MO system relative to the MB system. This was attributed to increased iron corrosion by virtue of formation of soluble Fe2+-MO complexes. The overall result questions the usefulness of pre-treating (e.g. acid washing) Fe0 materials before testing in short term experiments and reiterates the importance of longer lasting experiments with Fe0 materials.
Graphical abstract Download high-res image (169KB)Download full-size image