| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 575320 | Journal of Hazardous Materials | 2016 | 8 Pages |
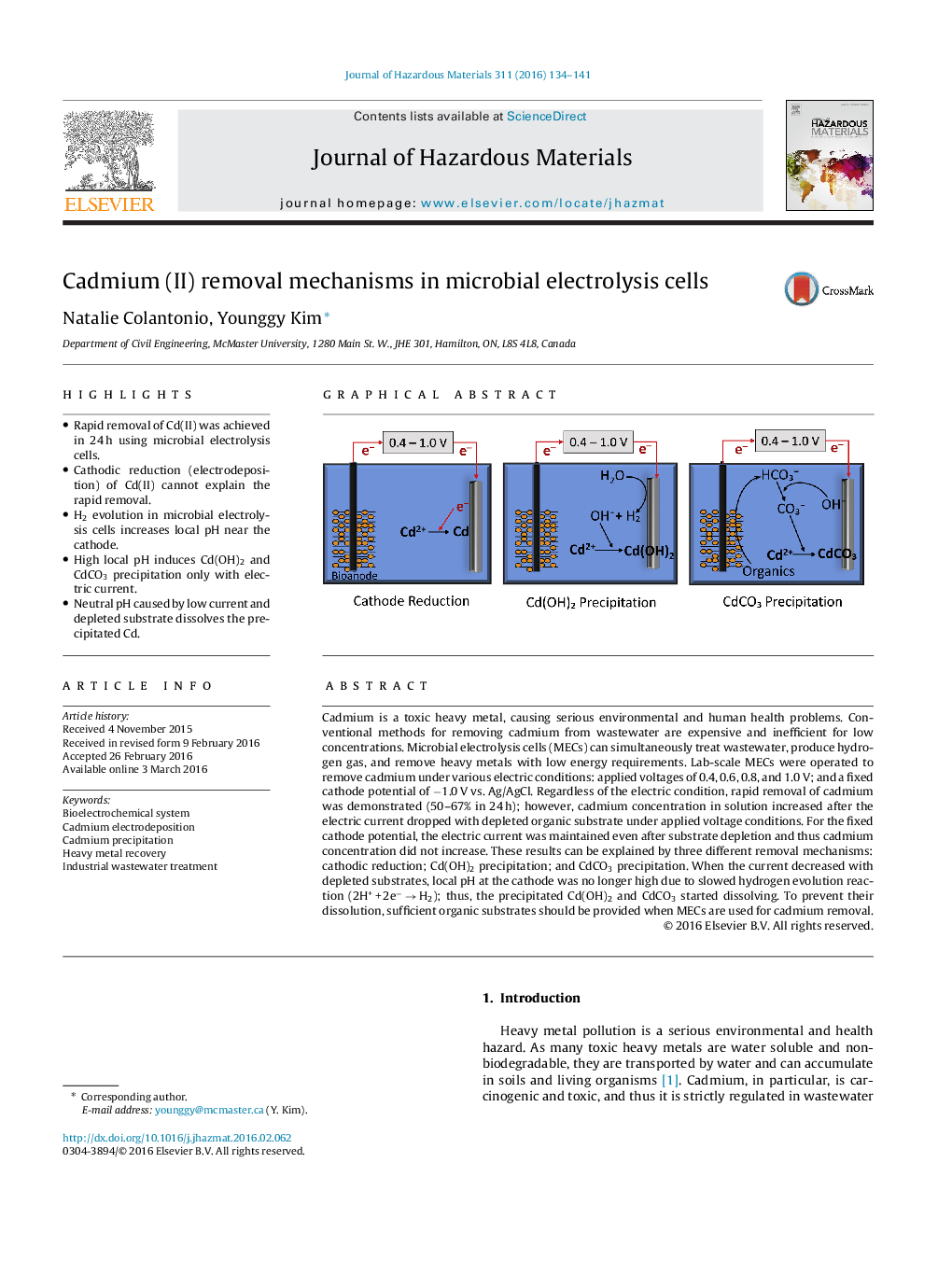
•Rapid removal of Cd(II) was achieved in 24 h using microbial electrolysis cells.•Cathodic reduction (electrodeposition) of Cd(II) cannot explain the rapid removal.•H2 evolution in microbial electrolysis cells increases local pH near the cathode.•High local pH induces Cd(OH)2 and CdCO3 precipitation only with electric current.•Neutral pH caused by low current and depleted substrate dissolves the precipitated Cd.
Cadmium is a toxic heavy metal, causing serious environmental and human health problems. Conventional methods for removing cadmium from wastewater are expensive and inefficient for low concentrations. Microbial electrolysis cells (MECs) can simultaneously treat wastewater, produce hydrogen gas, and remove heavy metals with low energy requirements. Lab-scale MECs were operated to remove cadmium under various electric conditions: applied voltages of 0.4, 0.6, 0.8, and 1.0 V; and a fixed cathode potential of −1.0 V vs. Ag/AgCl. Regardless of the electric condition, rapid removal of cadmium was demonstrated (50–67% in 24 h); however, cadmium concentration in solution increased after the electric current dropped with depleted organic substrate under applied voltage conditions. For the fixed cathode potential, the electric current was maintained even after substrate depletion and thus cadmium concentration did not increase. These results can be explained by three different removal mechanisms: cathodic reduction; Cd(OH)2 precipitation; and CdCO3 precipitation. When the current decreased with depleted substrates, local pH at the cathode was no longer high due to slowed hydrogen evolution reaction (2H+ + 2e− → H2); thus, the precipitated Cd(OH)2 and CdCO3 started dissolving. To prevent their dissolution, sufficient organic substrates should be provided when MECs are used for cadmium removal.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide