| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 591444 | Colloids and Surfaces A: Physicochemical and Engineering Aspects | 2016 | 10 Pages |
•Al(OH)3-Fads and Al(OH)3-Fcoag can effectively adsorb As(III), As(V), and Cd(II).•More significant decrease of SBET and pHiep is observed at higher AT.•Incorporated-F inhibit the removal of Cd and As as compared to pristine Al(OH)3.•Elevated AT benefit Cd adsorption whereas inhibit the removal of As(III) and As(V).•Annealing these aluminas at elevated AT may control fluoride leaching control.
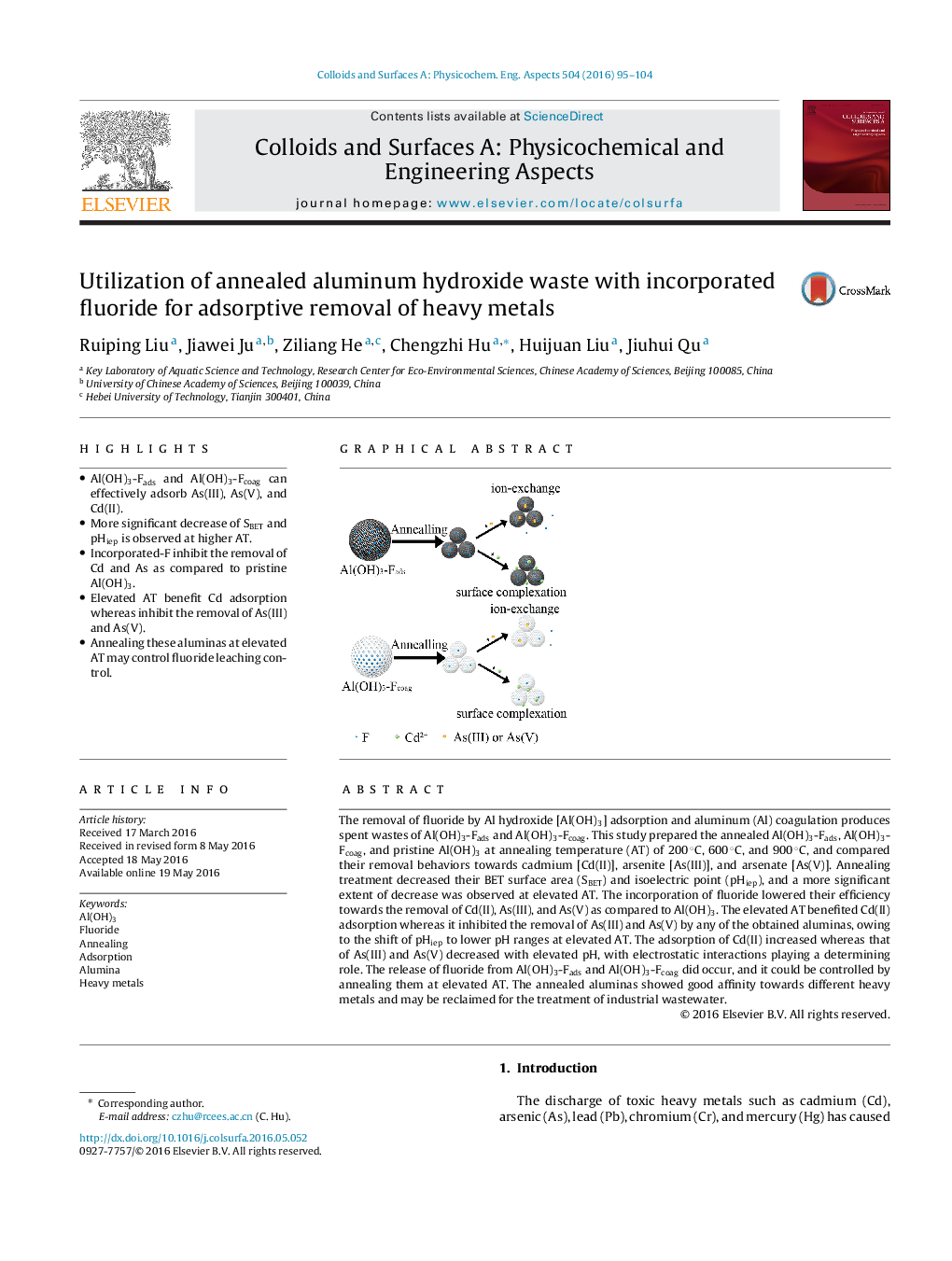
The removal of fluoride by Al hydroxide [Al(OH)3] adsorption and aluminum (Al) coagulation produces spent wastes of Al(OH)3-Fads and Al(OH)3-Fcoag. This study prepared the annealed Al(OH)3-Fads, Al(OH)3-Fcoag, and pristine Al(OH)3 at annealing temperature (AT) of 200 °C, 600 °C, and 900 °C, and compared their removal behaviors towards cadmium [Cd(II)], arsenite [As(III)], and arsenate [As(V)]. Annealing treatment decreased their BET surface area (SBET) and isoelectric point (pHiep), and a more significant extent of decrease was observed at elevated AT. The incorporation of fluoride lowered their efficiency towards the removal of Cd(II), As(III), and As(V) as compared to Al(OH)3. The elevated AT benefited Cd(II) adsorption whereas it inhibited the removal of As(III) and As(V) by any of the obtained aluminas, owing to the shift of pHiep to lower pH ranges at elevated AT. The adsorption of Cd(II) increased whereas that of As(III) and As(V) decreased with elevated pH, with electrostatic interactions playing a determining role. The release of fluoride from Al(OH)3-Fads and Al(OH)3-Fcoag did occur, and it could be controlled by annealing them at elevated AT. The annealed aluminas showed good affinity towards different heavy metals and may be reclaimed for the treatment of industrial wastewater.
Graphical abstractFigure optionsDownload full-size imageDownload as PowerPoint slide