| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 61502 | Journal of Catalysis | 2012 | 7 Pages |
Three rhodium catalysts supported on rare earth oxides with redox properties, Rh/CeO2, Rh/Pr6O11, and Rh/Tb4O7, were tested for their ability to trigger oxidative reforming of n-butane at room temperature. Only Rh/CeO2 triggered the oxidative reforming, owing to the heat generated by the spontaneous oxidation of the CeO2−x species produced by prior reduction of the supported catalyst with H2. Although the three rare earth oxides were reduced to CeO1.91, Pr2O3, and Tb2O3, respectively, by H2 at ⩾873 K, CeO1.91 was the only reduced oxide that was oxidized upon exposure to O2 at room temperature. These results indicate that the ability of the reduced oxide support to undergo oxidation at room temperature was crucial for triggering oxidative reforming of n-butane at room temperature.
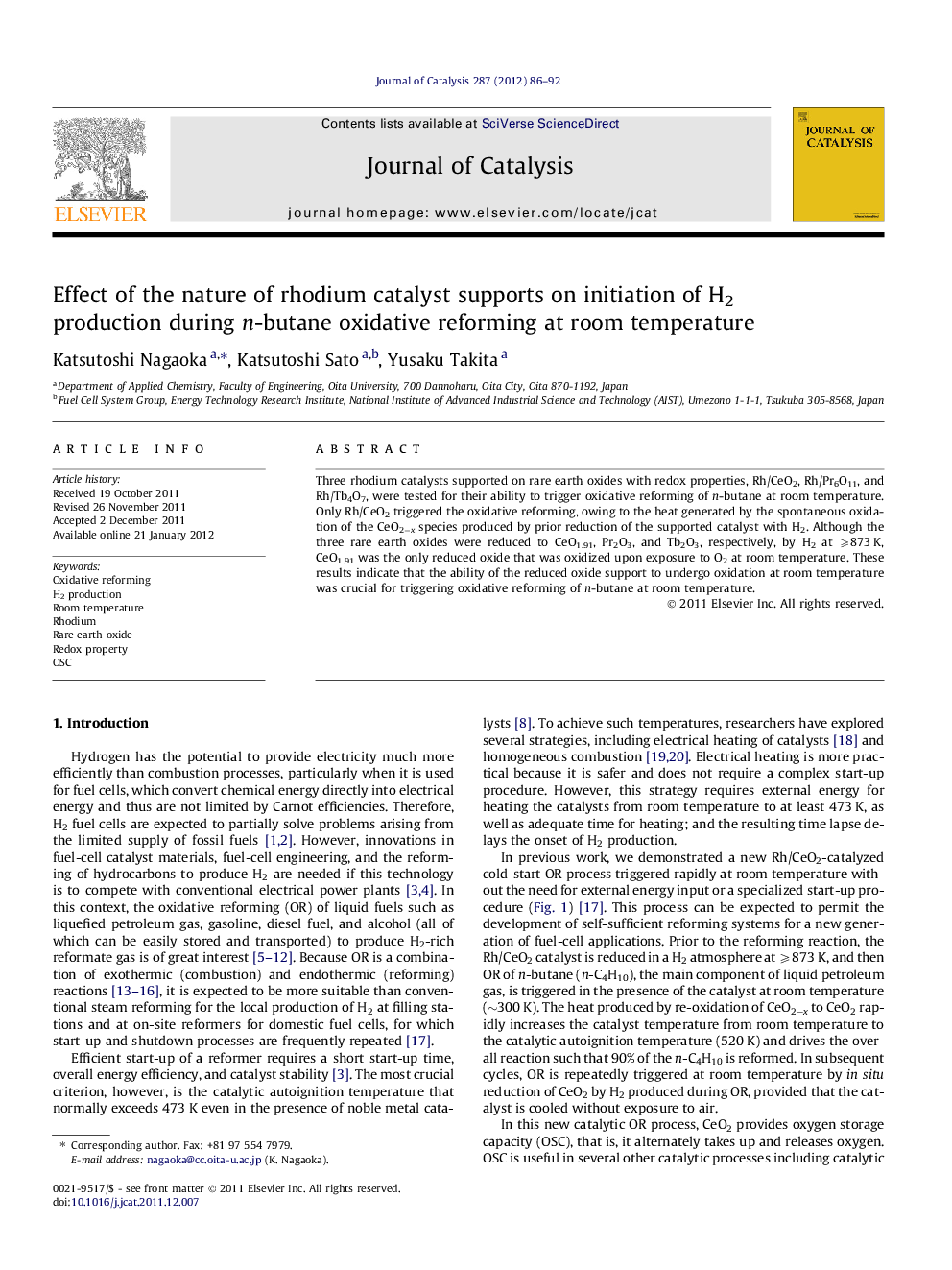
Graphical abstractOxidative reforming of n-butane was triggered rapidly at room temperature over Rh/CeO2−x but not over Rh/Pr2O3 and Rh/Tb2O3. The unique ability of CeO2−x, unlike Pr2O3 and Tb2O3, to be oxidized at room temperature played a crucial role in triggering oxidative reforming at room temperature.Figure optionsDownload full-size imageDownload high-quality image (104 K)Download as PowerPoint slideHighlights► Rh/CeO2−x enabled triggering oxidative reforming of n-butane at room temperature. ► Rh/Pr6O11 and Rh/Tb4O7 could not trigger the reaction at room temperature. ► Rh/CeO2−x was oxidized at room temperature. ► Rh/Pr2O3 and Rh/Tb2O3 were not oxidized at room temperature. ► The oxidation ability of CeO2−x played a crucial role for triggering the reaction.