| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 62742 | Journal of Catalysis | 2008 | 13 Pages |
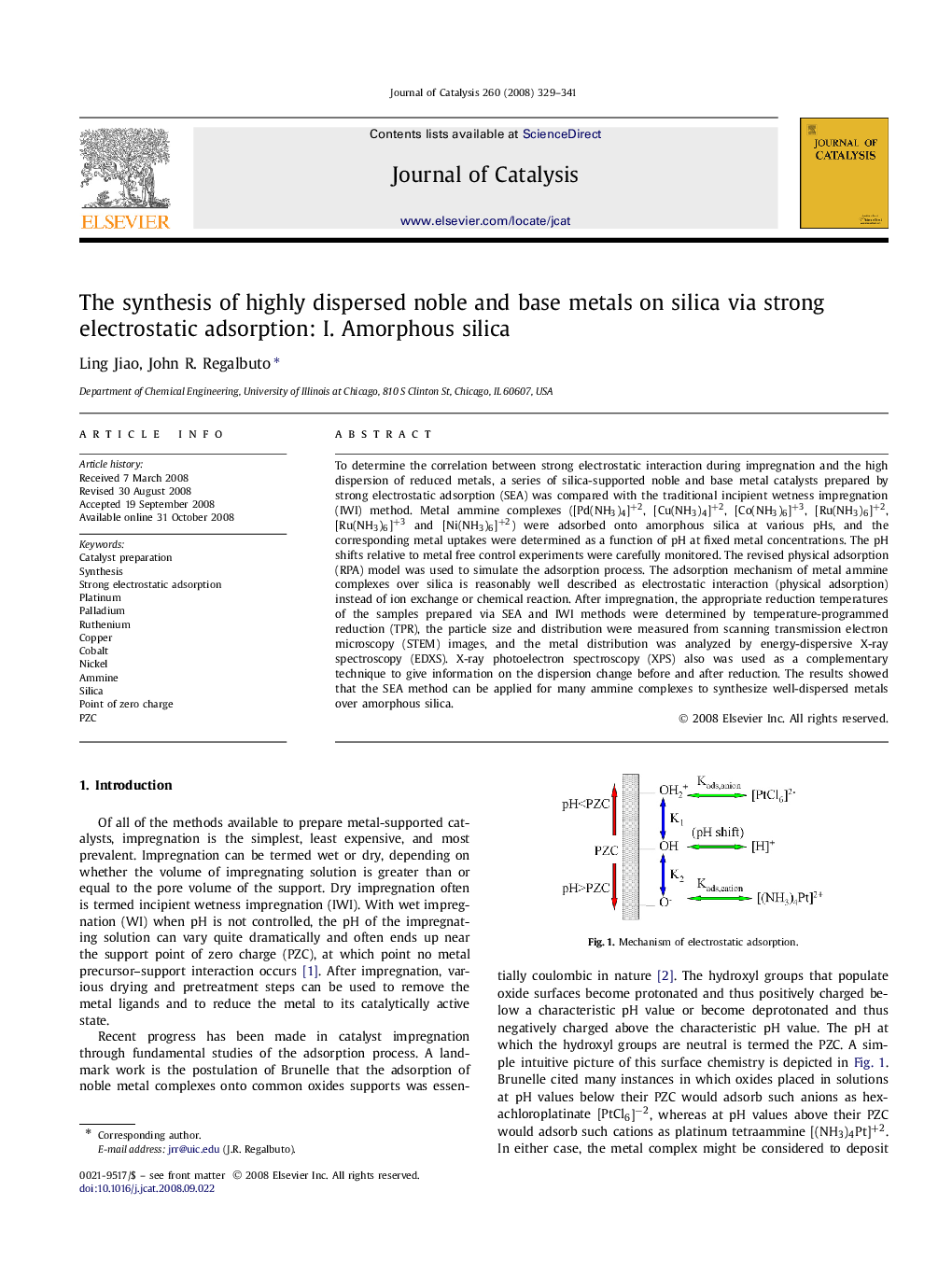
To determine the correlation between strong electrostatic interaction during impregnation and the high dispersion of reduced metals, a series of silica-supported noble and base metal catalysts prepared by strong electrostatic adsorption (SEA) was compared with the traditional incipient wetness impregnation (IWI) method. Metal ammine complexes ([Pd(NH3)4]+2, [Cu(NH3)4]+2, [Co(NH3)6]+3, [Ru(NH3)6]+2, [Ru(NH3)6]+3 and [Ni(NH3)6]+2) were adsorbed onto amorphous silica at various pHs, and the corresponding metal uptakes were determined as a function of pH at fixed metal concentrations. The pH shifts relative to metal free control experiments were carefully monitored. The revised physical adsorption (RPA) model was used to simulate the adsorption process. The adsorption mechanism of metal ammine complexes over silica is reasonably well described as electrostatic interaction (physical adsorption) instead of ion exchange or chemical reaction. After impregnation, the appropriate reduction temperatures of the samples prepared via SEA and IWI methods were determined by temperature-programmed reduction (TPR), the particle size and distribution were measured from scanning transmission electron microscopy (STEM) images, and the metal distribution was analyzed by energy-dispersive X-ray spectroscopy (EDXS). X-ray photoelectron spectroscopy (XPS) also was used as a complementary technique to give information on the dispersion change before and after reduction. The results showed that the SEA method can be applied for many ammine complexes to synthesize well-dispersed metals over amorphous silica.