| Article ID | Journal | Published Year | Pages | File Type |
|---|---|---|---|---|
| 6309208 | Chemosphere | 2014 | 8 Pages |
â¢Carbon and hydrogen isotope fractionation during multistep sorption was measured for benzene and toluene.â¢Successive hydrophobic partitioning steps result in low isotope fractionation.â¢We observed carbon and hydrogen isotope fractionation for the benzene-octanol pair.â¢Functional groups of SOM may specifically interact with BTEX compounds.
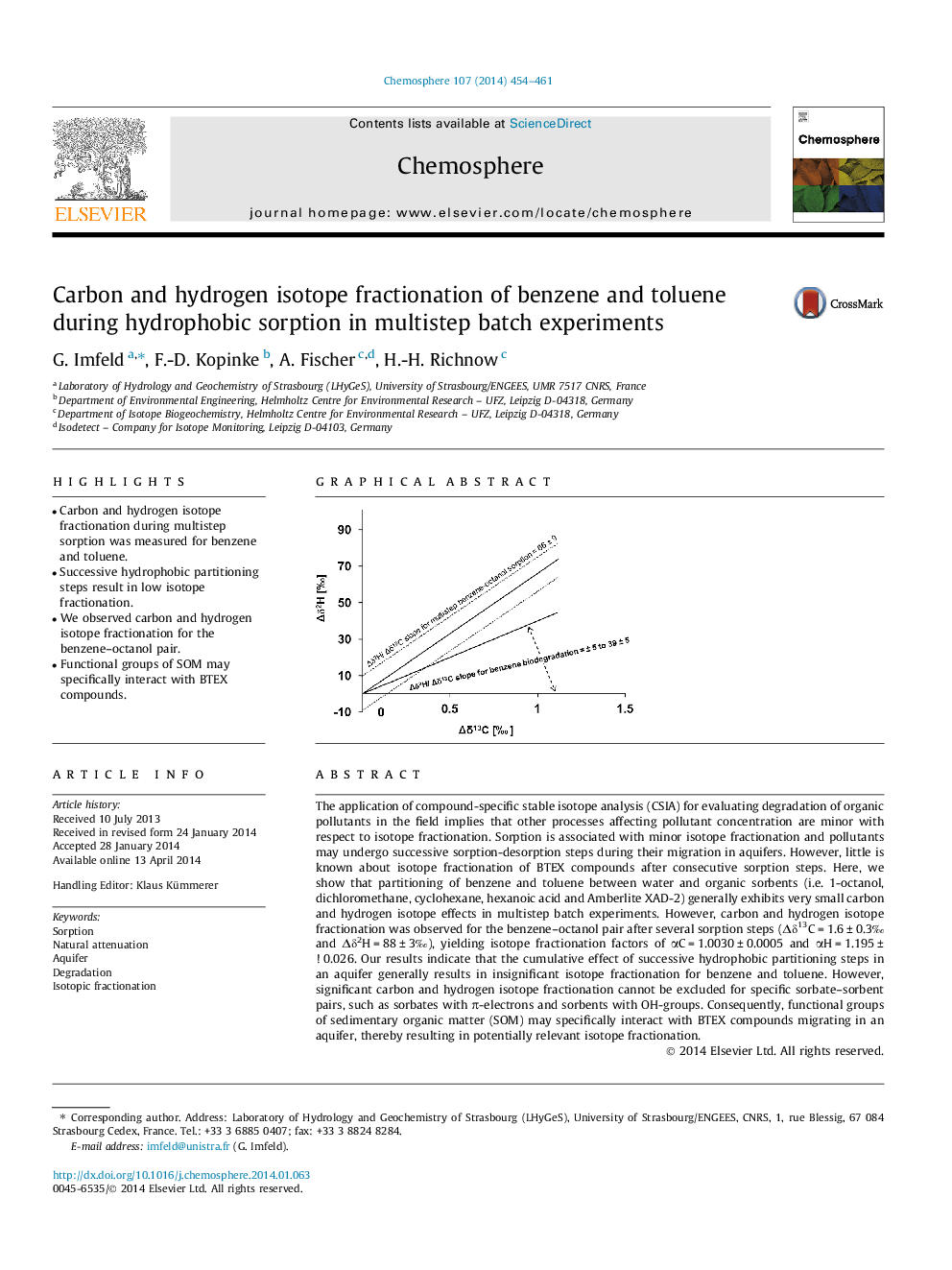
The application of compound-specific stable isotope analysis (CSIA) for evaluating degradation of organic pollutants in the field implies that other processes affecting pollutant concentration are minor with respect to isotope fractionation. Sorption is associated with minor isotope fractionation and pollutants may undergo successive sorption-desorption steps during their migration in aquifers. However, little is known about isotope fractionation of BTEX compounds after consecutive sorption steps. Here, we show that partitioning of benzene and toluene between water and organic sorbents (i.e. 1-octanol, dichloromethane, cyclohexane, hexanoic acid and Amberlite XAD-2) generally exhibits very small carbon and hydrogen isotope effects in multistep batch experiments. However, carbon and hydrogen isotope fractionation was observed for the benzene-octanol pair after several sorption steps (Îδ13C = 1.6 ± 0.3â° and Îδ2H = 88 ± 3â°), yielding isotope fractionation factors of αC = 1.0030 ± 0.0005 and αH = 1.195 ± 0.026. Our results indicate that the cumulative effect of successive hydrophobic partitioning steps in an aquifer generally results in insignificant isotope fractionation for benzene and toluene. However, significant carbon and hydrogen isotope fractionation cannot be excluded for specific sorbate-sorbent pairs, such as sorbates with Ï-electrons and sorbents with OH-groups. Consequently, functional groups of sedimentary organic matter (SOM) may specifically interact with BTEX compounds migrating in an aquifer, thereby resulting in potentially relevant isotope fractionation.
Graphical abstractDownload full-size image